-
PROVIDERS
New MRD Medicare Coverage for Select Indications*
*When coverage criteria are met. Additional criteria and exceptions for coverage may apply.
-
LIFE SCIENCES
REGISTER NOW
UPCOMING WEBINAR
Driving enterprise value with RWD -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
07/17/2025
Lower Checkpoint Gene Expression is Associated with Primary Resistance to Nivolumab-Ipilimumab Combination in Advanced Renal Cell Carcinoma
KCRS 2025
PRESENTATION
Authors
Rana McKay, Adam Dugan, Unnati Jariwala, Karyn Ronski, Jacob Mercer, Eddy Saad, Jad El Masri, Marc Machaalani, Mustafa Saleh, Cody Rose, Maxine Sun, Sabina Signoretti, David Braun, Sumanta Pal, Toni Choueri
Background – Immunotherapy combinations have emerged as the standard of care for patients with advanced renal cell carcinoma (RCC). While the combination of first-line (1L) nivolumab and ipilimumab has demonstrated long-term durability with 90-month progression-free survival (PFS) of 23%, primary progressive disease (PD) occurs in up to 20% of patients. There is an urgent clinical need to identify negative selection markers for patients unlikely to respond to nivolumabipilimumab therapy.
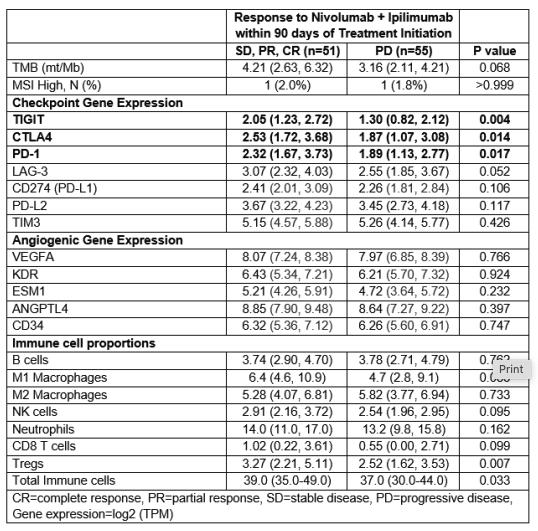
Methods – We used Tempus Lens to select records for 106 patients with RCC who received 1L ipilimumab-nivolumab treatment from the Tempus multi-modal database. Eligible patients had tumor samples collected within one year of treatment initiation and were processed using xT (DNA-seq) and xR (RNA-seq) assays. Treatment response was investigator-assessed within 90 days of therapy start and categorized as progressive disease (PD) versus non-PD, which includes stable disease, partial response, or complete response. RNA-seq data were normalized, log2-transformed, and batch-corrected and used to measure checkpoint and angiogenic gene expression (Transcripts per million, TPM). Immunologic phenotype was assessed by TMB (mt/Mb), MSI status, PD-L1 (IHC, 22c3); immune infiltration was estimated by quanTIseq (RNA).
Results – Of the 106 patients, 55 experienced PD as the best response. The cohort was predominantly male (73%) and white (86%), with a median age at diagnosis of 59 years. Clinical characteristics were similar between treatment response categories, including rates of clear cell RCC (82% vs. 76%, p=0.480) and primary nephrectomy (47% vs. 53%, p=0.560). Patients with primary PD were more likely to have liver metastases (20% vs. 5.9%, p=0.032). There was no significant difference in PD-L1 (IHC) expression between groups (p=0.542). However, patients with primary PD exhibited significantly lower gene expression of CTLA4, TIGIT, and PD-1 as well as lower proportions of immune cells (Table 1). We observed a trend toward lower LAG3 expression and TMB in the PD group. Strong positive correlations were demonstrated between LAG3 expression and other checkpoint genes (all p<0.002). No significant differences were found in angiogenic gene expression or somatic tumor alterations between response groups.
Conclusions – In patients with advanced RCC treated with 1L nivolumabipilimumab, we identified distinct transcriptomic patterns of primary resistance characterized by lower expression of immune checkpoint genes. These findings suggest that tumors with reduced checkpoint pathway activity may be less responsive to dual checkpoint inhibition, possibly reflecting an immune-cold microenvironment with limited T-cell infiltration and activation. Future prospective validation of these findings, including IHC, could inform precision medicine strategies for patients with advanced RCC.