-
PROVIDERS
REGISTER NOW
Navigating new frontiers in breast cancer care pathway intelligence: The role of providers and AI
Tuesday, July 29th
2:00pm PT, 4:00pm CT, 5:00pm ET -
LIFE SCIENCES
REGISTER NOW
Closing Care Gaps with AI: The Next Competitive Edge in Pharma
Monday, July 14
9am PT, 11am CT, 12pm ET -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
RADIOLOGY /// THERAPY RESPONSE EVALUATION
AI-enabled solution to automatically measure and track lesion progression
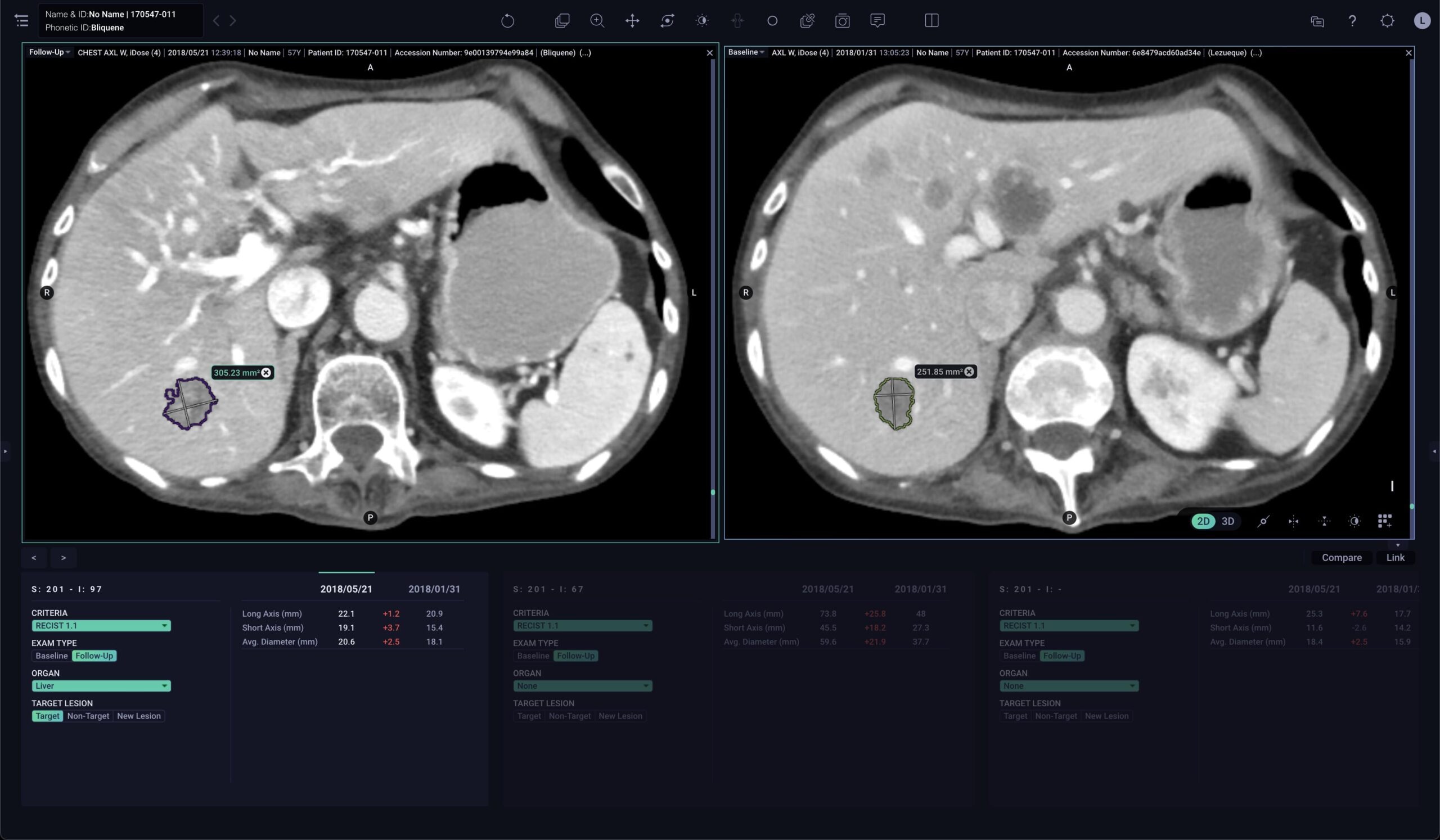
Tempus Pixel provides advanced analysis, tools, and automated reporting from radiology images to help providers accurately track, quantify, and efficiently make RECIST1 assessments and other clinical decisions.
Single-click lesion segmentation
Segment and measure lesions of interest within radiology images for efficient and consistent therapy response assessments2
Automated lesion tracking and reporting
Access longitudinal tracking reports that automatically calculates lesion response3,4
Seamless integration
Easily integrate with existing PACS and EHR systems to optimize radiology workflows
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. Doi: https://doi.org/10.1016/j.ejca.2008.10.026
- Tempus Pixel Therapy Response Evaluation single-click lesion segmentation feature is semi-automated and powered by DeepLook’s DL Precise (K202084) but is not available for clinical use in the EU. Arterys is the manufacturer of Tempus Pixel, excluding any third party components described in this list. DeepLook is the manufacturer of DL Precise. DL Precise is not CE marked.
- In the U.S., Tempus Pixel Lung is FDA-cleared (K203744) but is not indicated for lung nodule detection. Lung nodule automated detection and quantification is powered by InferRead CT (K192880). Arterys Inc is the manufacturer of Tempus Pixel Lung, excluding any third party components described in this list. Infervision is the manufacturer of InferRead CT.
- In the EU, Tempus Pixel Lung is CE marked and indicated for lung nodule detection. RECIST is pending CE mark. Arterys Inc is the manufacturer of Tempus Pixel Lung.