TEMPUS SEQUENCING FOR RESEARCHERS
Tempus offers a broad range of contract and traditional clinical options using our CAP-accredited CLIA-certified next-generation sequencing labs.
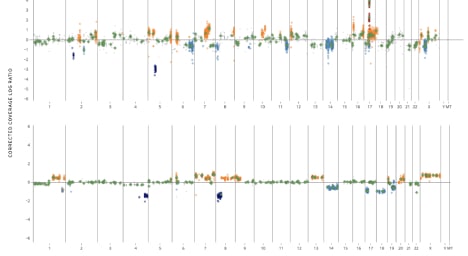
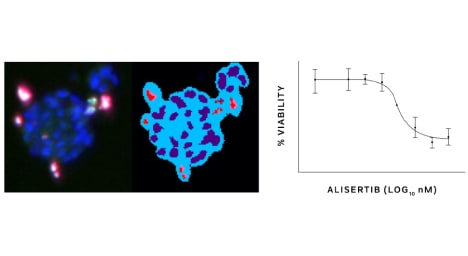
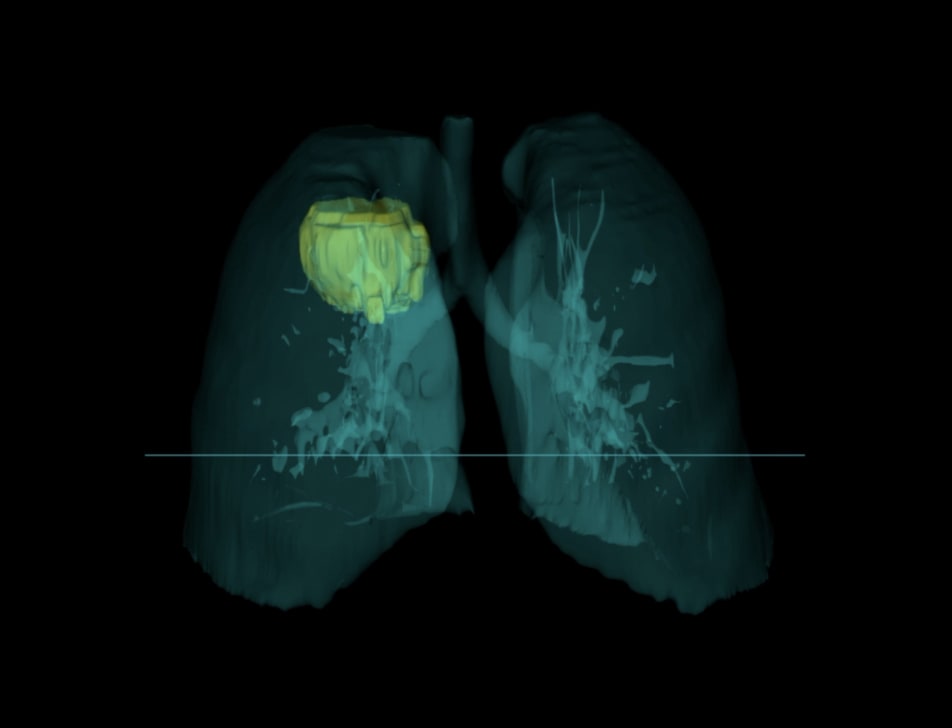
Tempus has additional capabilities including single-cell sequencing, genome-wide liquid biopsy profiling, spatial transcriptomics, methylation, genotyping and more.
Learn more about our sequencing capabilities