-
PROVIDERS
New MRD Medicare Coverage for Select Indications*
*When coverage criteria are met. Additional criteria and exceptions for coverage may apply.
-
LIFE SCIENCES
Learn more
Building the engine to scale data abstraction through AI
Learn how Tempus’ AI engine transforms unstructured clinical text into analysis-ready real-world data -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
12/18/2025
A new data-driven playbook for improving ADC success rates
ADCs hold immense promise but face significant development hurdles, from patient selection to acquired resistance. A platform combining multimodal RWD, AI-driven biomarker discovery, and PDOs can de-risk the entire ADC lifecycle and improve PTS.
Authors
Andy Moye
SVP & GM, Data Products, Tempus

SVP & GM, Data Products, Tempus

Confronting the late-stage challenge in ADC development
In my role at Tempus, I’ve had a front-row seat to the transformation of RWD in oncology, and the era of ADCs is one of the most exciting developments. These therapies offer a powerful new modality for delivering cytotoxic payloads directly to cancer cells. Yet, I’ve also seen firsthand that the path from lab to clinic is fraught with challenges.
In conversations with life sciences companies, I often reference a key statistic: while ADCs have a higher overall preclinical-to-approval success rate than other oncology drugs, they paradoxically face lower success rates in later clinical phases; only about 24% of ADCs entering Phase 3 actually achieve success.1
This late-stage attrition points to fundamental complexities in ADC development that we must solve. At Tempus, we believe that overcoming these challenges requires a new paradigm—an integrated, data-driven flywheel that connects real-world patient biology to preclinical models and back again, de-risking the development pipeline at every step.
|
Explore how Tempus’ integrated platform of real-world data, AI, and organoid modeling can help accelerate and de-risk antibody-drug conjugate development. |
The complexity of ADC development
The intricate design of ADCs, an antibody, a linker, and a payload, creates multiple points of potential failure. Three key challenges contribute to these late-stage setbacks:
- Precise patient selection: Historically, development has relied on the expression of a single target antigen. However, this often proves to be an insufficient biomarker, as patients with similar antigen levels can have vastly different clinical outcomes.
- Understanding mechanisms of resistance: The reasons why patients stop responding to treatment are often unclear. Characterizing this treatment-induced evolution at the molecular level is critical for developing next-generation ADCs and combination strategies.
- Predicting response heterogeneity: The heterogeneity of response that you actually see in the real world is not what you’re predicting in the lab. To bridge this gap, we must ground discovery and development in a comprehensive, multimodal understanding of the patient.
Building a foundation on real-world data and AI
Tempus’ platform leverages a large multimodal, de-identified real-world dataset to support research and development in oncology. By integrating clinical data with molecular data, including DNA sequencing, whole-transcriptome RNA sequencing, and digital pathology images, we provide a comprehensive view to support oncology research. This foundation supports life sciences companies in exploring key questions in ADC development during preclinical research.
Real-world data allows researchers to move beyond single-biomarker hypotheses. For example, by analyzing TROP2 expression across a large set of de-identified patient profiles, researchers can explore potential indications for anti-TROP2 ADCs.
Antibody selection is only half the story; you also have to understand your payload sensitivities. By mapping gene signatures and antigen expression in our real-world data, we support companies in exploring:
- Potential antigen-payload combinations for further research.
- Hypotheses for combination therapies.
- Methods for patient stratification based on molecular profiles.
RNA sequencing provides a quantitative measure of gene expression, which may offer additional insights for patient stratification compared to immunohistochemistry (IHC).
|
“This is the foundation of precision medicine, and it is how we can deliver on the full promise of ADCs for patients.“ – Andy Moye, SVP & GM, Data Products, Tempus |
Validating insights with patient-derived models
Real-world data generates powerful hypotheses, but preclinical validation is essential. Tempus Loop is a platform that integrates real-world data, patient-derived organoids (PDOs), and AI to support hypothesis testing in preclinical models.
A recent project Tempus worked on illustrates this power. For the Nectin-4 ADC enfortumab vedotin, Nectin-4 expression alone is a relatively poor predictor of response. Using our library of PDOs, which undergo the same comprehensive molecular profiling as our clinical patients, our team identified a novel 30-gene RNA signature associated with response.
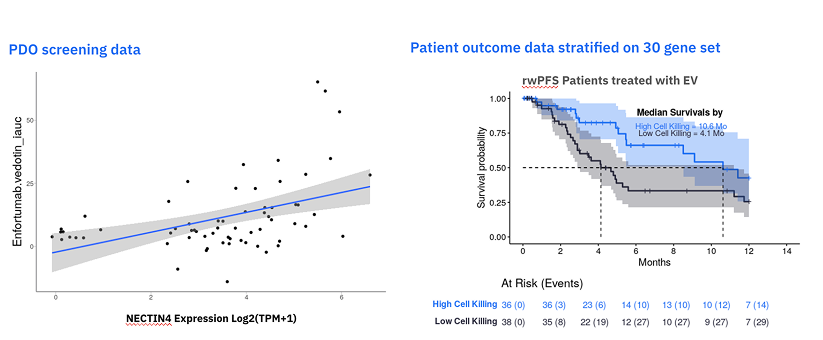
Internal Tempus database analysis. September 2025.
We then validated this signature in our real-world data. The results were clear:
- In a cohort of patients treated with the drug, the 30-gene signature successfully stratified individuals by progression-free survival.
- In a cohort of patients who were not treated with the drug, the signature showed no ability to separate outcomes.
This suggests that the AI-discovered signature may be associated with a response to a specific therapy, based on retrospective analysis. This is the kind of work that we get excited about at Tempus—using AI, real-world data, and patient-derived organoids to help identify these kinds of biomarkers.→
Accelerating clinical trials and commercialization
Finding the right patients for clinical trials: Enrolling patients in ADC trials can be a significant bottleneck, especially when eligibility depends on a novel or hard-to-access test. We help solve this challenge through just-in-time clinical trial matching. By applying AI models to Tempus xR whole-transcriptome data, we developed and validated unique algorithmic models to predict a patient’s likelihood of having a positive IHC result for key biomarkers, including HER2, TROP2, and Nectin-4, thereby facilitating potential clinical trial enrollment.
Closing care gaps post-launch: Once an ADC is on the market, identifying and testing patients who may be eligible for treatment can be a major hurdle. The Tempus Next Oncology platform integrates with hospital EMRs and uses AI-enabled tools to identify patients who may meet guideline-based criteria for biomarker testing. In one instance, this approach supported an institution in increasing its EGFR testing rate. Tempus can work with you to implement similar care gap tools in EHRs to optimize testing in a way that helps ensure the right patients are treated with ADCs.
The complexity of ADC development demands an equally sophisticated solution. An integrated approach that combines the scale of real-world data, the biological fidelity of patient-derived models, and the power of AI can create a virtuous cycle of discovery and validation. By grounding research in real-world patient biology, we can identify more robust biomarkers, design more effective clinical trials, and ultimately improve the probability of technical and regulatory success. This is the foundation of precision medicine, and it is how we can deliver on the full promise of ADCs for patients.
1. Beacon ADC Database. Beacon by handsonwade; https://beacon-intelligence.com/solutions/adc-database/.
| To learn more about how Tempus can help de-risk your ADC pipeline and accelerate development, contact our life sciences team here. |
-
02/12/2026
Beyond the EHR: Building a reliable mortality endpoint for oncology research
Emilie Scherrer, Senior Director and Head of Outcomes Research, explains how Tempus’ validated, composite mortality endpoint overcomes data fragmentation to provide a trustworthy foundation for real-world evidence.
Read more -
02/05/2026
Accelerating a phase 1 oncology trial: The TIME Network’s impact on patient enrollment
For life sciences companies, speed and precision are paramount. The ability to efficiently launch a Phase 1 trial and generate early clinical data is fundamental to long-term success. The Tempus TIME Trial Network provides the infrastructure to help achieve these goals.
Read more -
02/05/2026
Building the engine to scale data abstraction through AI
Learn how Tempus’ AI engine, built on agentic architectures, transforms unstructured clinical text into analysis-ready real-world data. Tempus AI leaders detail the multi-layered system that ensures speed, scale, and fidelity.
Read more


