-
PROVIDERS
New MRD Medicare Coverage for Select Indications*
*When coverage criteria are met. Additional criteria and exceptions for coverage may apply.
-
LIFE SCIENCES
Learn more
Building the engine to scale data abstraction through AI
Learn how Tempus’ AI engine transforms unstructured clinical text into analysis-ready real-world data -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
Tempus xH
Whole genome sequencing (WGS) assay
Tempus xH is an analytically validated1 next-generation sequencing (NGS) test that uses a WGS approach for the detection of actionable oncologic targets in peripheral blood and/or bone marrow samples from target populations with hematopoietic malignancies.
The performance of the Tempus xH assay was established in an analytical validation study using 235 unique specimens, including whole blood, bone marrow, and cell lines. The results were compared against established orthogonal methods to determine the assay’s accuracy and precision.2
Assay specifications
-
Depth of sequencing
Targeting 80X mean autosomal coverage.
-
Platforms used
Illumina NovaSeq X Plus.
-
Accepted sample types
Blood or bone marrow (BM).
-
Alterations identified
SNVs, Indels, copy number alterations (CNAs ≥5 Mb), and structural variants (SVs).
-
Sensitivity
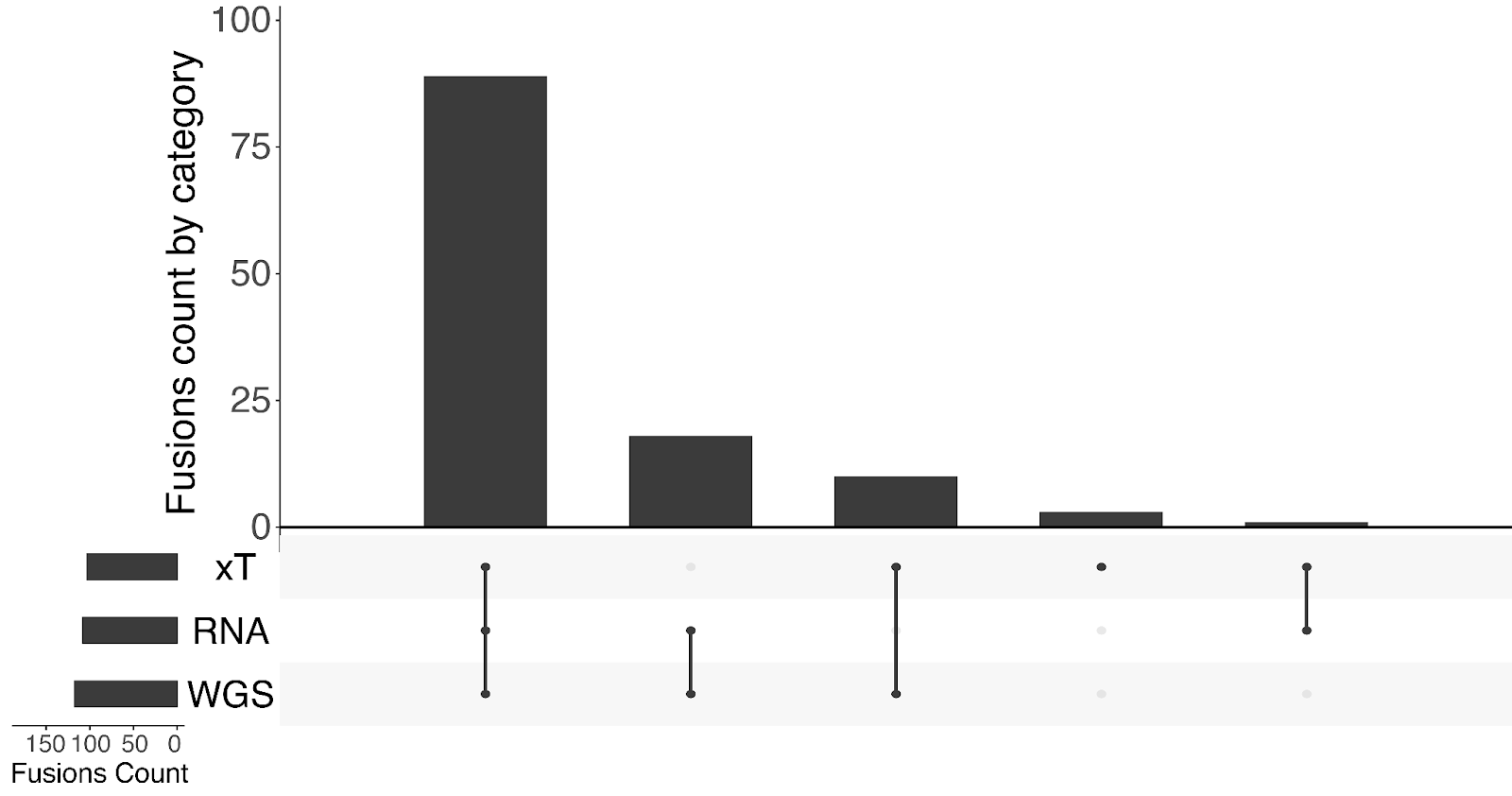
Demonstrated high positive percent agreement (PPA) when compared to orthogonal methods: 97.29% for SNVs/Indels, 92.86% for SVs, and 93.43% for CNAs.²
xH was developed for use in Acute Myeloid Leukemia (AML), Myelodysplastic Syndromes (MDS), Myeloproliferative Neoplasms (MPN), and overlap syndromes (MDS/MPN). It is optimized for myeloid neoplasms and supports disease understanding, biomarker discovery, and therapeutic research.
By scaling WGS-based testing for myeloid malignancies, Tempus supports life science researchers to drive innovation, accelerate therapeutic discovery, and shape the future of precision medicine.
DETECTION AND CONCORDANCE
High accuracy compared to orthogonal methods
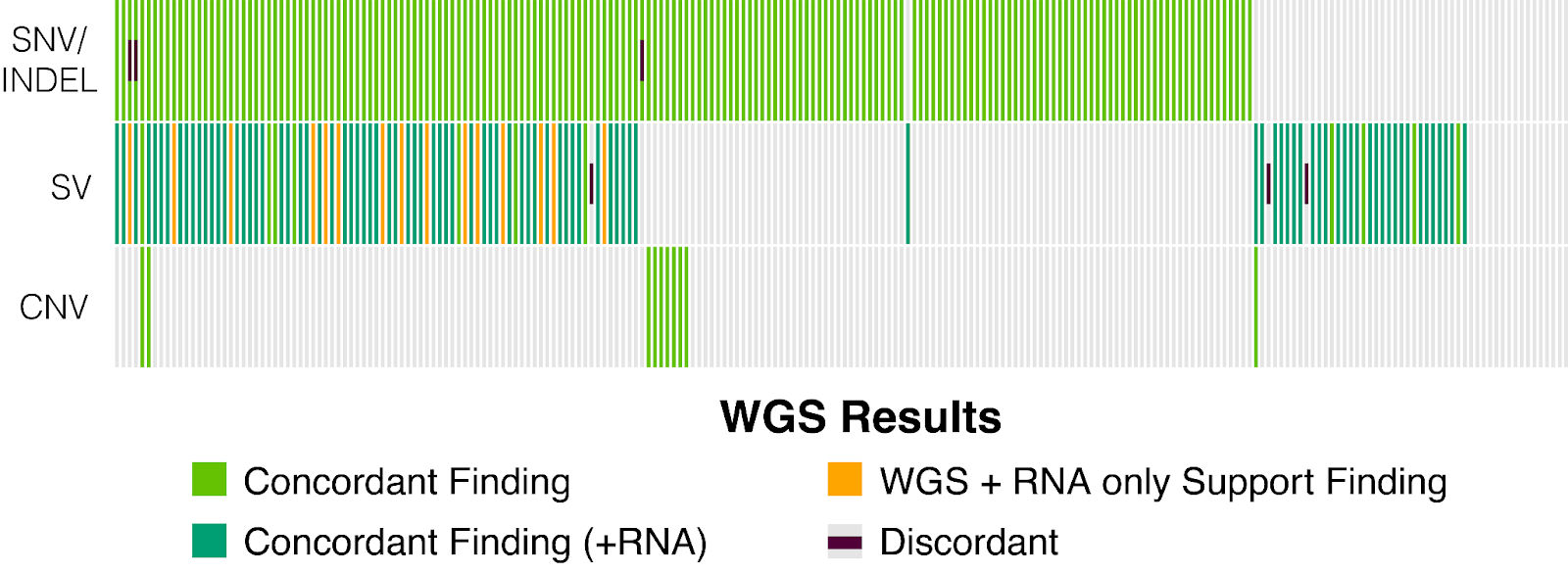
The analytical validation of Tempus xH demonstrated high accuracy across all major alteration types when compared to established orthogonal methods. The assay achieved 97.29% PPA for SNVs and Indels against the Tempus xT assay, 92.86% PPA for SVs against the Tempus xR assay and Sanger sequencing, and 93.43% PPA for CNAs against an Oligo-SNP chromosomal microarray.²
RESULTS
Reliable and precise results
In addition to high accuracy, the xH assay demonstrated excellent precision, ensuring reliable and reproducible results. For SNVs and Indels, the assay achieved a positive predictive value (PPV) of 98.97%, and for SVs, it achieved a PPV of 100%. This high level of precision minimizes false positives and provides confidence in the detected genomic alterations.²
Learn more about the launch of our xH assay in this press release
Read now
- Analytically validated in a CLIA certified laboratory.
- Internal data on file.
This is data-driven precision medicine
This is the future of healthcare.