-
PROVIDERS
New MRD Medicare Coverage for Select Indications*
*When coverage criteria are met. Additional criteria and exceptions for coverage may apply.
-
LIFE SCIENCES
Register now
UPCOMING WEBINARTranslating data into an actionable R&D strategy
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
01/05/2026
Baseline Biomarker Analysis and Clinical Outcomes of the PD-1/TGFβR2 Bispecific Antibody INCA33890 in Patients With Non-MSI-H Metastatic Colorectal Cancer (mCRC)
ASCO GI 2026
PRESENTATION
Authors
Justin T. Moyers, Michelle Kinder, Rui Hong, Michael Smith, Martin Gutierrez, Sreenivasa R. Chandana, Markus Joerger, Irene Moreno, Victor Moreno, Armando Santoro, Debra H. Josephs, Haeseong Park, Massimo A. Di Nicola, Tatiana Hernandez Guerrero, Jordi Rodon Ahnert, Maria Vieito Villar, Xiaohan Xu, Chiara Greggio, Thomas B. Karasic, Ruth Plummer
Background:INCA33890 is an anti–PD-1/TGFβR2 bispecific antibody designed to antagonize TGFβR2/PD-1 signaling in immune cells co-expressing both targets. In the INCA33890-101 study (NCT05836324), patients (pts) with refractory non-MSI-H mCRC were treated with INCA33890 monotherapy at 3 expansion doses. Responses were observed in pts with and without active liver metastases. Here, we analyze associations between baseline biomarkers and clinical outcomes.
Methods:Fresh or archival (≤3 years from study registration) baseline biopsies were required in the dose expansion part and assessed using immunohistochemistry (IHC) for CD8 and PD-L1 (SP263) and whole transcriptome and next-generation sequencing for DNA aberrations (both Tempus xT). Baseline ctDNA was analyzed using Predicine Atlas during dose escalation and expansion. Efficacy was assessed by investigators per RECIST v1.1.
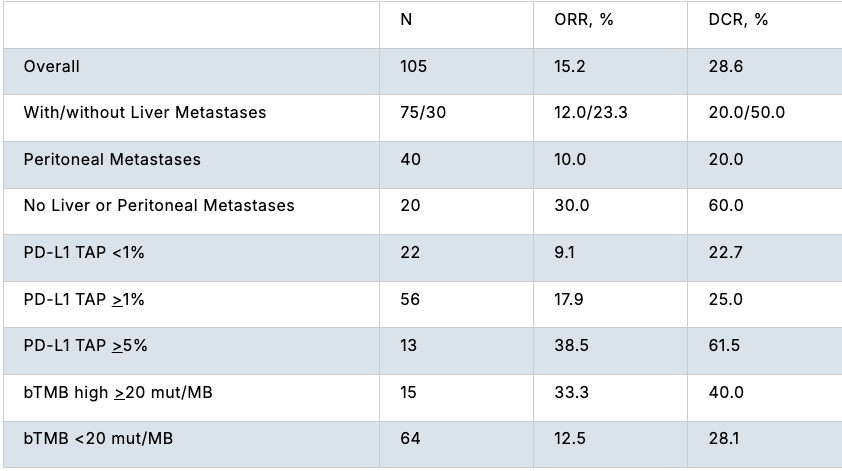
Results:As of July 25, 2025, efficacy data with INCA33890 monotherapy were available from 105 pts; 16 pts (15.2%) had a partial response (PR), including pts with liver and peritoneal metastases (Table). Of the 78 pts with evaluable baseline biopsies for IHC analysis, pts with PR or stable disease (SD) showed greater PD-L1 tumor area positivity scores than those with progressive disease (PD) (nominal p<0.05). mRNA expression of inflammatory genes (IFN-γ, CXCL9, CXCL10 and T-cell inflamed signature) were higher in pts with PR/SD vs PD. Pts with blood tumor mutation burden (bTMB) >20 mut/MB had a trend towards higher ORR (33.3%) vs pts with bTMB <20 mut/MB (12.5%). Consensus molecular subtypes (CMS) were assessed in 44 pts. Although CMS 4 is commonly associated with the presence of TGF-β, it was not associated with a higher ORR (7.7% [1/13]). Responses were also observed in CMS 2 (21.7% [5/23]) and CMS 1 (50% [1/2]), but not in CMS 3 (n=6). There was no relationship with tumor mRNA expression of TGFβ or TGFβ-associated signatures with clinical outcomes. In addition, SMAD4 mutations, determined by ctDNA, were present in both responders and non-responders. Mutations in KRAS (64%), TP53 (72%), and APC (47%) were the most common aberrations and had no impact on clinical outcomes.
Conclusions:INCA33890 demonstrated preliminary efficacy in pts with non-MSI-H mCRC across a variety of subgroups. Higher baseline PD-L1 expression and mRNA expression of inflammatory genes enriched for clinical benefit while overexpression of TGF-β did not seem to enhance efficacy. Updated data will be presented.
Efficacy across subgroups.