-
PROVIDERS
New MRD Medicare Coverage for Select Indications*
*When coverage criteria are met. Additional criteria and exceptions for coverage may apply.
-
LIFE SCIENCES
Register now
UPCOMING WEBINARTranslating data into an actionable R&D strategy
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
RADIOLOGY /// PROSTATE
AI-enabled solution designed to revolutionize prostate MRI interpretation1

Tempus Pixel offers an AI-enabled solution that is trained with pathology data to detect suspicious lesions for clinically significant prostate cancer, and therefore can improve diagnostic accuracy and efficiency in aggressive prostate cancer detection and diagnosis. Pixel Prostate enhances prostate MRI interpretation by automating QC against PI-RADS v2.1 guidelines, gland segmentation, lesion detection and risk classification.
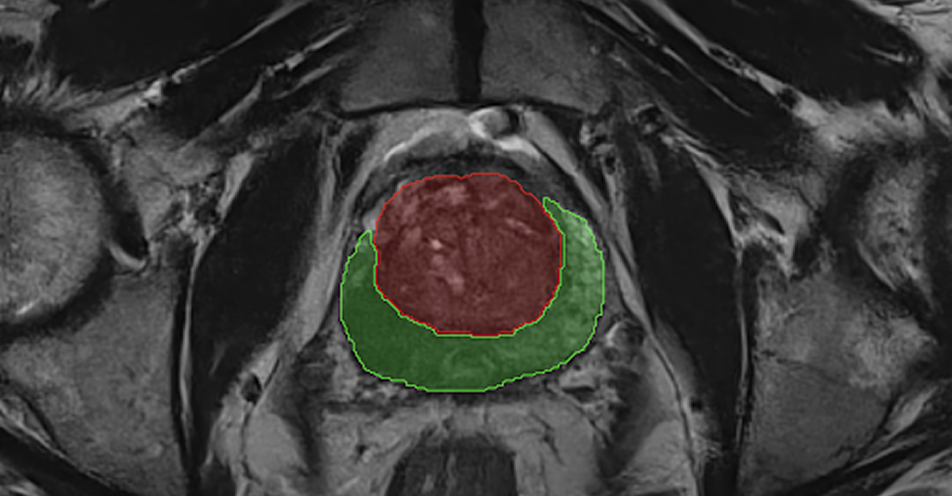
PROSTATE SEGMENTATION
Automatically segments the prostate zones, providing volume and dimensions.1
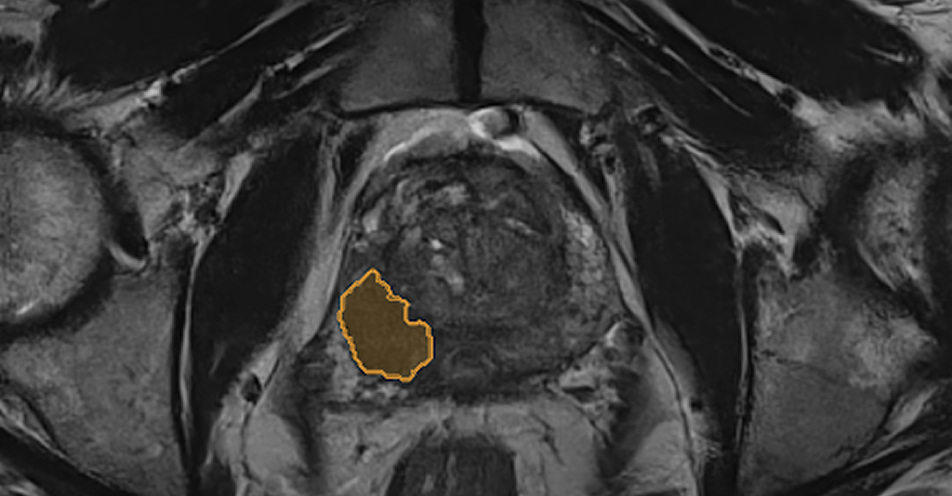
LESION DETECTION
Automatically detects suspicious lesions for clinically significant prostate cancer, and therefore can improve diagnostic accuracy of radiologists. 1,2
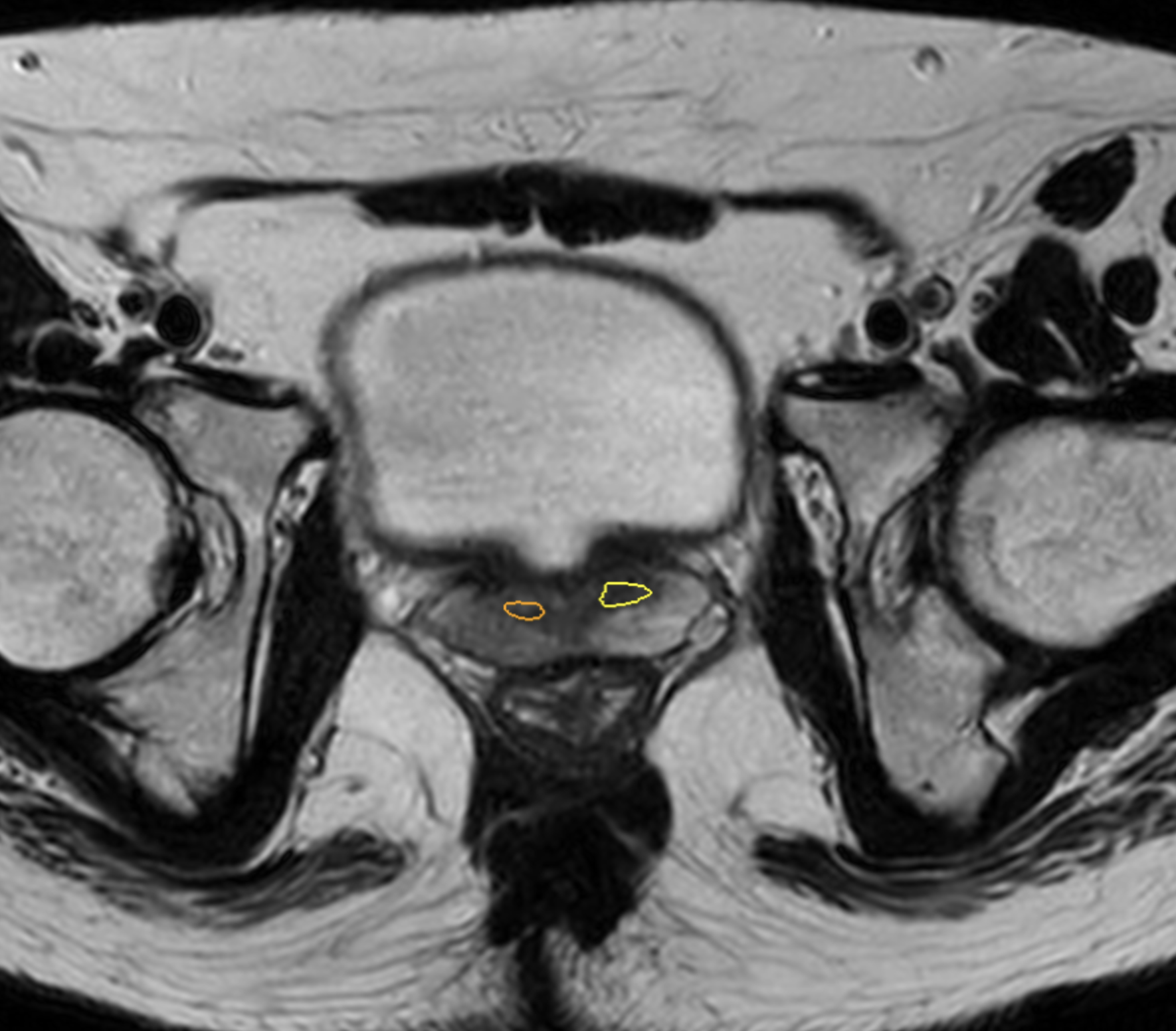
RISK CLASSIFICATION
Automatically classifies detected lesion as either high or moderate probability. 1
STREAMLINED WORKFLOW
Automatic QC and reporting, as well as seamless integration with PACS and EMR.1

- Tempus Pixel is FDA-cleared (K203744) and CE Marked. Prostate gland segmentation is powered by Quibim’s QP-Prostate® (K203582). QC against PI-RADS v2.1 guidelines is powered by Quibim’s QP-Prostate® (K203582) and QP-Prostate® CAD (K242683). Lesion detection and risk classification for clinically significant prostate cancer is powered by Quibim’s QP-Prostate® CAD (K242683). Arterys Inc is the manufacturer of Tempus Pixel. Quibim is the manufacturer of QP-Prostate® and QP-Prostate® CAD
- Quibim data on file. J. Hillis and S. Mercaldo, “Quibim QP-Prostate CAD: Standalone Model Performance Assessment and Clinical Reader Performance Assessment: Clinical Study Report (Clinical Reader),” Mass General Brigham, QUI-C-002-REP-002, 2024.