-
PROVIDERS
New MRD Medicare Coverage for Select Indications*
*When coverage criteria are met. Additional criteria and exceptions for coverage may apply.
-
LIFE SCIENCES
Learn more
Building the engine to scale data abstraction through AI
Learn how Tempus’ AI engine transforms unstructured clinical text into analysis-ready real-world data -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
11/20/2025
Q&A: Designing a modern CDx strategy for ADCs
Tempus experts discuss the essential pillars of a modern CDx strategy, from leveraging multimodal data to streamlined regulatory pathways.
Authors
Mike Yasiejko
Executive Vice President and
General Manager, Genomics

Lauren Silvis
Senior Vice President of
External Affairs

Executive Vice President and
General Manager, Genomics

Lauren Silvis
Senior Vice President of
External Affairs

The oncology pipeline is shifting toward novel therapeutics like antibody-drug conjugates (ADCs), creating a need for more sophisticated and integrated approaches to diagnostic development. In this Q&A, Mike Yasiejko, Executive Vice President and General Manager of Genomics, and Lauren Silvis, Senior Vice President of External Affairs at Tempus, discuss how a unified platform that leverages real-world data (RWD), advanced sequencing, and regulatory expertise can de-risk and accelerate the path to market for the next wave of cancer therapies.
The oncology pipeline is shifting towards novel therapeutics like ADCs. Why does this require a new approach to diagnostic development? |
Mike: The clinical trial landscape has seen a dramatic increase in novel therapeutics, with ADCs, multi-specific antibodies, and cell and gene therapies now comprising about a third of all trials.1 This creates a new set of challenges. Each new ADC may require its own separate immunohistochemistry (IHC) test, which is not a scalable solution. In clinical testing, tissue availability is frequently a limiting factor, and we cannot continue adding single-biomarker tests for every new drug.
This situation creates an urgent need for a more sophisticated diagnostic strategy. Because many of these antibody-based therapies target proteins that are overexpressed rather than mutated, RNA-based biomarkers are becoming essential. To support this new era, we need an infrastructure built on three core principles: a complete, multimodal picture of the patient from a single sample; more predictive tests built with sophisticated models; and a scalable platform to connect the right patient to the right therapy—from clinical trials through to commercial launch. |
|
“Ultimately, our value lies in providing an integrated, end-to-end partnership. We help [pharma] build a clear strategy using RWD and AI, leverage a unified platform to develop an accurate diagnostic, navigate the regulatory process, and finally, launch with immediate access to an established commercial channel.” – Mike Yasiejko, Executive Vice President and General Manager, Genomics, Tempus |
How can RWD help de-risk ADC development from the very beginning? |
Mike: It is critical to start with a foundation of RWD to set an effective strategy. The Tempus database contains multimodal data, including genomic, transcriptomic, and deeply curated clinical information, from thousands of patients treated with ADCs. This allows you to analyze response and resistance in a real-world context before a trial even begins.
Unlike public datasets like TCGA, which often focus on earlier-stage or untreated disease, our data includes later-stage, metastatic, and heavily pre-treated patients, directly reflecting the population enrolled in clinical trials. For example, by analyzing our data, we can see how biomarker expression, like HER2, changes after treatment or how expression of a gene, like SLFN11, differs between primary and metastatic sites. We can also see how a target like FOLR1 may be highly expressed in early-stage lung cancer but decreases significantly in metastatic disease, making it a riskier target than it might initially appear. These insights, which are only possible with deep, longitudinal RWD, allow for a more realistic and effective biomarker strategy. |
How does RNA sequencing compare to the traditional IHC standard for ADC companion diagnostics? |
Mike: A key part of a modern ADC strategy is moving beyond the limitations of IHC. While IHC is a trusted standard, our whole-transcriptome sequencing assay, xR, identifies a majority of the same patients. More importantly, RNA sequencing provides a robust, quantitative surrogate that can outperform IHC in predicting patient outcomes.
For example, in an internal analysis of over 800 breast cancer patients treated with trastuzumab deruxtecan (T-DXd), IHC could broadly separate the HER2 3+ patients but grouped the 0, 1+, and 2+ patients. In contrast, the continuous, quantitative nature of RNA data allowed us to stratify those same patients into at least three distinct outcome groups, providing a much more granular understanding of who is most likely to benefit. RNA can also be used to pre-screen patients and identify those most likely to be IHC-positive, which lowers screen-fail rates and accelerates trial enrollment, all while preserving precious tissue. |
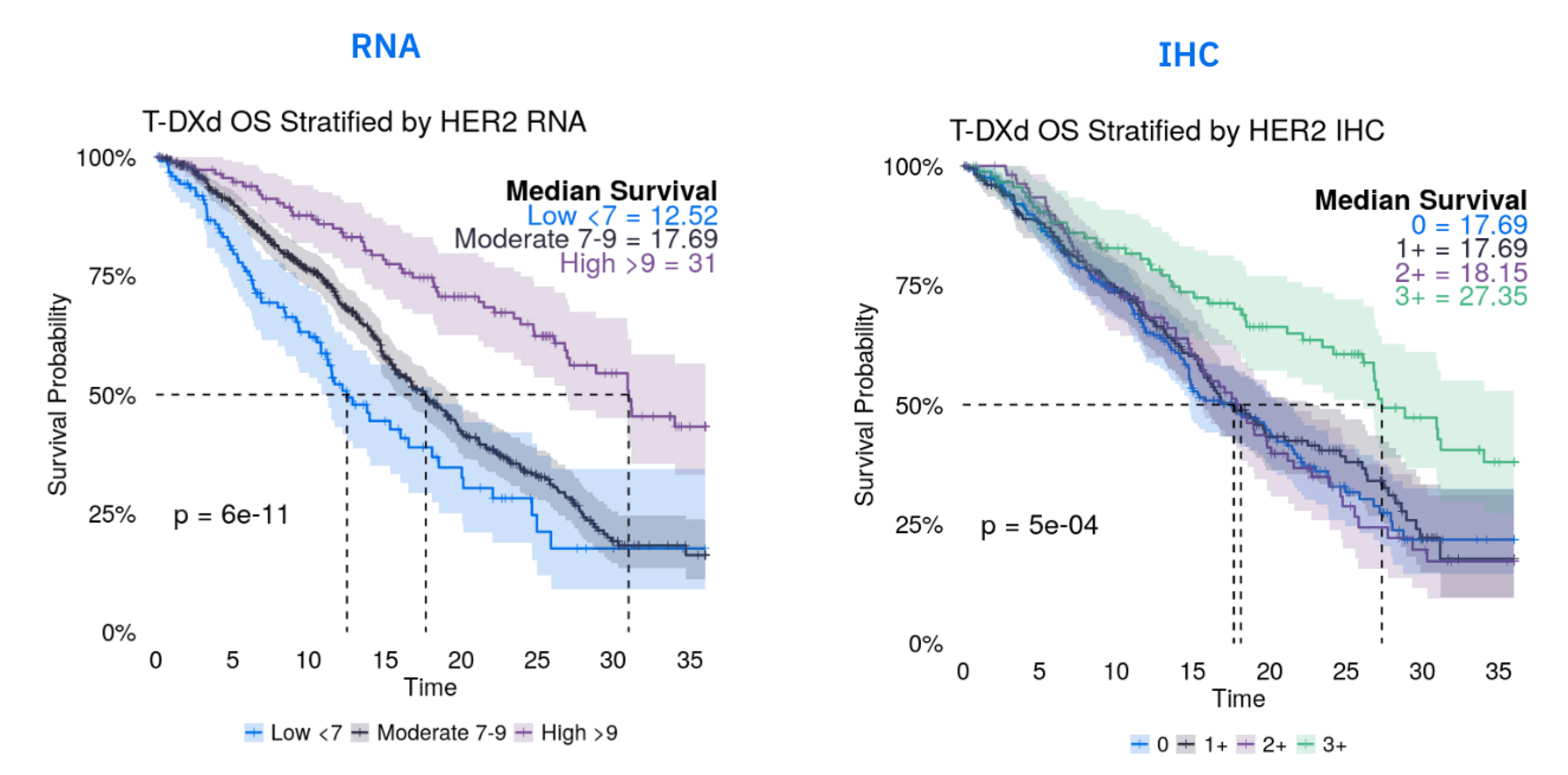
Internal Tempus database analysis. September 2025
|
“We have a team that includes former FDA staff, and we have successfully brought our own assays through the regulatory process, including our xT CDx DNA panel and our recently 510(k)-cleared RNA assay for RET and BRAF rearrangements.” – Lauren Silvis, Senior Vice President of External Affairs, Tempus |
Can you share a practical example of how this RNA-first screening approach is being used? |
Mike: We are putting this RNA-first approach into practice in the ADC-MATCH trial, which aims to test multiple ADC drugs across a pan-cancer patient population. The investigators initially struggled to identify and recruit eligible patients because of the need for IHC-positivity for targets that are not routinely tested, such as Nectin-4, creating a significant screening challenge.
To solve this, we used our RNA platform and IHC prediction algorithms to screen thousands of patients upfront. This allowed us to identify the small subset of patients most likely to be positive, who could then be sent for confirmatory IHC testing. This strategy is dramatically accelerating enrollment and demonstrates our ability to generate novel insights and quickly deploy them at scale. |
What is the current regulatory landscape for these diagnostics, and how does Tempus help partners navigate it? |
Mike: Although the FDA’s proposed rule that would have regulated laboratory-developed tests (LDTs) as medical devices was recently rescinded, the fundamental principle remains unchanged: If you are developing a novel therapy, you must bring a well-validated test to the FDA. You have to demonstrate that you are selecting the right patients for your trial and that physicians will be able to appropriately identify patients once the drug is launched.
At Tempus, we have extensive experience navigating these pathways. We have a team that includes former FDA staff, and we have successfully brought our own assays through the regulatory process, including our xT CDx DNA panel and our recently 510(k)-cleared RNA assay for RET and BRAF rearrangements. This hands-on experience allows us to guide our customers through the complexities of Investigational Device Exemptions (IDEs), Premarket Approvals (PMAs), and 510(k)s and ensure they are prepared for engagement with the agency. |
Can you expand on how Tempus’ role as a large clinical lab informs its partnerships? |
| Lauren: Our connection from the regulatory phase into the clinic and back again is fundamental to our approach. Because we are one of the largest sequencers of cancer patients in the United States, we are not just developing tests in a vacuum. The experience we gain from running hundreds of thousands of tests in a real-world clinical setting directly informs our regulatory strategies. |
Once a diagnostic and therapy are developed, what role does Tempus play in commercialization and market adoption? |
| Mike: Our operational scale provides a distinct advantage for commercialization. Upon launch, you gain access to the Tempus network, which includes more than 6,500 oncologists and data from over 4 million cancer patients. We operate one of the largest genomics labs in the U.S., delivering over 270,000 next-generation sequencing tests last year alone, so we are well-equipped to support any commercial volume.
Ultimately, our value lies in providing an integrated, end-to-end partnership. We help pharmaceutical companies build a clear strategy using RWD and AI, leverage a unified platform to develop an accurate diagnostic, navigate the regulatory process, and finally, launch with immediate access to an established commercial channel. This integrated approach helps de-risk your program and accelerate your path to market. To gain comprehensive insights from the full discussion, we invite you to watch the presentation recording below. For in-depth demonstrations of our AI-enabled applications, contact us here. |
Note: Content edited for clarity. Please note that the content in this document has been revised for clarity and conciseness. Some language and formatting may have been adjusted to enhance readability while preserving the original meaning and intent of the discussion.
1. IQVIA Institute for Human Data Science. Global Oncology Trends 2025: Outlook to 2029. IQVIA. Published May 26, 2025. Accessed September 3, 2025. https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/global-oncology-trends-2025.
| To learn more about Tempus’ data solutions, click here or contact us. |
-
02/12/2026
Beyond the EHR: Building a reliable mortality endpoint for oncology research
Emilie Scherrer, Senior Director and Head of Outcomes Research, explains how Tempus’ validated, composite mortality endpoint overcomes data fragmentation to provide a trustworthy foundation for real-world evidence.
Read more -
02/05/2026
Accelerating a phase 1 oncology trial: The TIME Network’s impact on patient enrollment
For life sciences companies, speed and precision are paramount. The ability to efficiently launch a Phase 1 trial and generate early clinical data is fundamental to long-term success. The Tempus TIME Trial Network provides the infrastructure to help achieve these goals.
Read more -
02/05/2026
Building the engine to scale data abstraction through AI
Learn how Tempus’ AI engine, built on agentic architectures, transforms unstructured clinical text into analysis-ready real-world data. Tempus AI leaders detail the multi-layered system that ensures speed, scale, and fidelity.
Read more


