-
PROVIDERS
New MRD Medicare Coverage for Select Indications*
*When coverage criteria are met. Additional criteria and exceptions for coverage may apply.
-
LIFE SCIENCES
REGISTER NOW
Ask a Scientist:
How to leverage novel datasets to answer complex research questions -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
Tempus IO™
A comprehensive immunotherapy platform that provides actionable and cutting-edge services for life sciences partners advancing IO therapies, including antibody-drug conjugates (ADCs), TCR therapies, and immune checkpoint inhibitors (ICIs).
An all-in-one biomarker platform
Comprehensive real-world data (RWD)
~99% of whole transcriptome profiles contain at least one IO biomarker
Robust suite of IO biomarkers
10+ IO biomarkers available through Tempus sequencing
Advanced analytical tech
Leverage AI- and ML-based analytical tools to unlock actionable insights
Immune Profile Score (IPS)
First-of-its-kind biomarker utilizing DNA and RNA to prognosticate ICI response
Insights delivered with every sample
Assess key aspects of the tumor microenvironment (TME) and understand resistance mechanisms using standard and novel biomarker data. Tempus IO data is included with applicable next-generation sequencing (NGS) assay orders to ensure comprehensive insights without the need for additional sample collection.
Download the Tempus IO overview to learn more-
Tempus xT: Solid Tumor DNA Seq
Tumor Mutational Burden (TMB)
Microsatellite Instability (MSI)
Neoantigen Prediction
Human Leukocyte Antigen (HLA) Typing
HLA Loss of Heterozygosity (LOH)
Oncogenic Virus
Mismatch Repair (MMR) Proteins by IHC1
PD-L1 Protein quantification by IHC1
Mechanisms of Tumor Resistance to Immunotherapy2
Immune Profile Score (IPS): ICI response prognosticator (available with concurrent xT and xR orders)
-
Tempus xE: Whole Exome
Tumor Mutational Burden (TMB)
Microsatellite Instability (MSI)
Neoantigen Prediction
Human Leukocyte Antigen (HLA) Typing
Oncogenic Virus
Mismatch Repair (MMR) Proteins by IHC1
PD-L1 Protein quantification by IHC1
Mechanisms of Tumor Resistance to Immunotherapy2
-
Tempus xR: Whole Transcriptome RNA Seq
Gene Expression Target Profiling3
Neoantigen Prediction
Immune Infiltration
Oncogenic Virus
T-cell Receptor / B-cell Receptor Repertoire
Mismatch Repair (MMR) Proteins by IHC1
PD-L1 Protein quantification by IHC1
Immune Profile Score (IPS): ICI response prognosticator (available with concurrent xT and xR orders)
-
Tempus xF/xF+: Liquid Biopsies
Blood Tumor Mutational Burden (bTMB) (xF+ only)
Microsatellite Instability (MSI) high status (xF/xF+)
Tumor Mutational Burden (TMB)
Microsatellite Instability (MSI)
Neoantigen Prediction
Human Leukocyte Antigen (HLA) Typing
HLA Loss of Heterozygosity (LOH)
Oncogenic Virus
Mismatch Repair (MMR) Proteins by IHC1
PD-L1 Protein quantification by IHC1
Mechanisms of Tumor Resistance to Immunotherapy2
Immune Profile Score (IPS): ICI response prognosticator (available with concurrent xT and xR orders)
Tumor Mutational Burden (TMB)
Microsatellite Instability (MSI)
Neoantigen Prediction
Human Leukocyte Antigen (HLA) Typing
Oncogenic Virus
Mismatch Repair (MMR) Proteins by IHC1
PD-L1 Protein quantification by IHC1
Mechanisms of Tumor Resistance to Immunotherapy2
Gene Expression Target Profiling3
Neoantigen Prediction
Immune Infiltration
Oncogenic Virus
T-cell Receptor / B-cell Receptor Repertoire
Mismatch Repair (MMR) Proteins by IHC1
PD-L1 Protein quantification by IHC1
Immune Profile Score (IPS): ICI response prognosticator (available with concurrent xT and xR orders)
Go beyond sequencing with powerful analytics
Tempus’ analytical capabilities leverage AI and machine learning to enhance sequencing data, enabling the identification of potential biomarkers that correlate with therapy response. Our proprietary tools uncover novel cancer dependencies and drug targets, particularly in IO-resistant populations. Tempus also offers supplementary data, custom analyses, and specialized algorithms as add-ons to meet your specific research objectives.4
Discover Tempus Lens for in-depth data and insights
Understand ICI treatment response
Utilize Tempus’ Immune Profile Score (IPS), a first-of-its-kind multimodal algorithm that uses DNA and RNA to provide prognostic insights into potential outcomes following immune checkpoint inhibitor (ICI) treatment in metastatic solid tumors.
The algorithm generates a score that categorizes study participants into:
- IPS-High: Indicates a higher likelihood of favorable outcomes with ICI treatment.
- IPS-Low: Indicates a lower likelihood of favorable outcomes with ICI treatment.
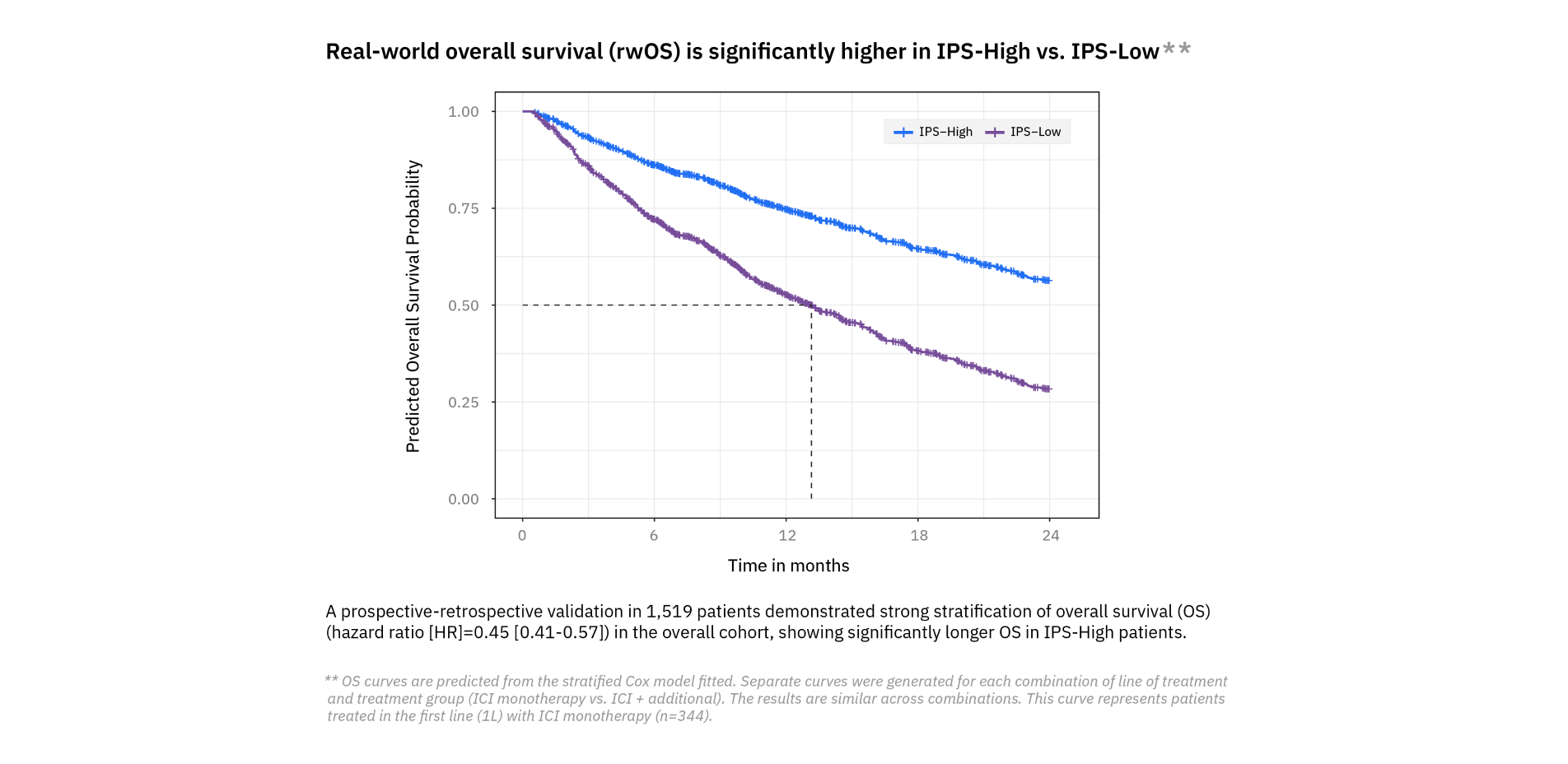
Powering clinical development with data and technology
Tempus operates CAP-accredited, CLIA-certified robotic sequencing labs in Chicago, Atlanta, and Raleigh with automated bioinformatics and variant classification reporting. Sequencing is typically completed within a median of 8 days after receiving samples.
Download our lab overview
-
UPCOMING WEBINAR:
Whole Genome Sequencing Uncovers Novel BCR::ABL1 Breakpoints and Variants In Leukemia: Implications for Personalized Medicine
An analysis of hematological samples used Tempus xH and xR assays to detect BCR::ABL1 fusions. This identified novel breakpoints with clinical implications, including one that suggests asciminib resistance, demonstrating the importance of WGS in personalized medicine.
Read now -
UPCOMING WEBINAR:
A Multi-Omic Immune Profile Score (IPS) Stratifies Real-World Outcomes of Microsatellite Stable (MSS) Advanced Colorectal Cancer Patients Treated With Immune Checkpoint Inhibitors
An exploratory study evaluated the multi-omic IPS as an ICI biomarker in a real-world cohort of advanced MSS CRC patients. IPS-High patients showed clinically meaningful prolonged rwOS on ICI, suggesting IPS utility as an ICI-specific predictive biomarker.
Read now -
UPCOMING WEBINAR:
MAIT Cell Abundance Within Solid Tumors Is Associated With Key Clinical Characteristics
Screening of multiregional patient-derived GTOs across glioma subtypes revealed marked variability in drug response between patients and across tumor regions, suggesting the need for multi-agent therapy and highlighting GTO utility for personalized treatment.
Read now -
UPCOMING WEBINAR:
Ultrahigh Tumor Mutational Burden (TMB) Is Associated With Improved Survival Outcomes in Patients (Pts) Treated With Immune Checkpoint Inhibitors (ICIs)
An analysis of ICI-treated patients identified Ultrahigh TMB as a novel marker for ICI response. This group showed significantly higher rwORR and improved rwOS, characterized by a higher infiltration of T cells and myeloid cells, supporting its utility for predicting therapy benefit.
Read now -
UPCOMING WEBINAR:
Longitudinal Changes in Circulating Tumor DNA in a Phase I Dose Escalation Study of Micvotabart Pelidotin, a First-in-Human ADC Targeting EDB+FN
A study assessed the ADC Micvotabart pelidotin MICVO in advanced solid tumors. Using the Tempus xF+ assay, a pharmacodynamic response was found: HNSCC patients showed significant reductions in ctDNA TF and bTMB by C3D1, supporting ctDNA utility as a liquid biomarker.
Read now -
UPCOMING WEBINAR:
Real-World Analyses To Evaluate the Role of TIGIT as a Target in First-Line (1L) Metastatic Non-Small Cell Lung Cancer (mNSCLC)
An analysis of patients using Tempus xR and PD-L1 IHC assessed TIGIT. TIGIT expression correlated strongly with Teff genes but poorly with PD-L1 status, suggesting PD-L1 alone may limit identification of responders to anti-TIGIT therapies.
Read now
- Assessment of MMR proteins by IHC, including MLH1, MSH2, MSH6, and PMS2, as well as PD-L1 IHC, is available as an optional add-on for Tempus xT/xE orders. This service is not automatically included in every order and should be selected by the customer.
- Tempus provides information on multiple variants and features that have been reported to confer resistance to immunotherapy. This includes mutations and/or copy number alterations in immune-associated genes that affect antigen presentation or interferon signaling. (xT, xE only)
- Tempus performs RNA whole transcriptome sequencing when sufficient tissue samples are provided.
- The availability of supplementary data is dependent on the scope of the request.
Trusted by hundreds of biopharma to power drug development
The information on this page is intended for life sciences companies and focuses on research and development applications.
Partner with us
With a broad range of laboratory services and data analysis that support immunotherapy development, Tempus has the solutions you need.