-
PROVIDERS
New MRD Medicare Coverage for Select Indications*
*When coverage criteria are met. Additional criteria and exceptions for coverage may apply.
-
LIFE SCIENCES
REGISTER NOW
Ask a Scientist:
How to leverage novel datasets to answer complex research questions -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
02/16/2023
Q&A: Using RWE for CDx and drug development
Leaders from Kronos Bio discuss how they use RWD from heterogeneous sources to strengthen companion diagnostic and drug development strategies.
Authors
Michael Yasiejko
General Manager and Executive Vice President, Tempus

Charles Lin, PhD
Senior Vice President, Biology, Kronos Bio

Pavan Kumar, PhD
Vice President, Translational Development, Kronos Bio

General Manager and Executive Vice President, Tempus

Charles Lin, PhD
Senior Vice President, Biology, Kronos Bio

Pavan Kumar, PhD
Vice President, Translational Development, Kronos Bio

This article is the first in our series on innovative approaches to companion diagnostic development. Look out for the next article in which we explore how our peers and collaborators are working to advance data-driven precision medicine.
From its inception, Kronos Bio has understood the imperative for real-world data (RWD) in developing transformative therapies for patients with cancer. As a clinical-stage biotech that specializes in addressing dysregulated transcription – which occurs when genes’ normal functions are disrupted, thereby causing a range of diseases and dysfunctions – Kronos uses heterogeneous data sources to understand the biology of tumors at the patient level.
Through a partnership with Tempus, Kronos is exploring how to integrate real-world evidence (RWE) across the drug development lifecycle, from discovery to regulatory filing. During the 2022 Precision Medicine Leaders Summit, Kronos leaders Charles Lin, PhD, and Pavan Kumar, PhD, spoke with Michael Yasiejko, MBA, General Manager and Executive Vice President at Tempus, to discuss how RWE impacts their companion diagnostic and drug development strategies. We’ve summarized their conversation below.
We’ve edited questions and responses for clarity and length.
Mike: Can you please describe your roles and backgrounds?
|
|
Dr. Lin: I’m the senior vice president of biology at Kronos. I’m a computational biologist by training and a former academic. My work at Kronos focuses on understanding and targeting dysregulated transcription in cancerous molecules. |
|
Dr. Kumar: I’m the vice president of translational development at Kronos. I’m a plant biologist by training and now focus on assay development and molecular biology. I’m responsible for making Charles’ ideas a reality in the clinic. |
Mike: How have you designed your RWD strategy, and what learnings have you gained that has reinforced your approach?
|
|
Dr. Lin: When bringing a new drug to the clinic, the ultimate goal is to improve patients’ lives. But at a tactical and molecular level, that’s actually really difficult. The best way to frame our goals and decisions is by letting the patient data guide us. |
|
Dr. Kumar: It’s critical to base any efforts in the pre-clinical phase on real patient data. I’ve seen teams try to use immortalized cell lines or other historical information to inform patient selection strategies, but these models don’t always reflect real tumors and real patients. This led to challenges in the clinic and catalyzed a pivot toward RWE. Data can also help define biomarker-high populations. Two common challenges to this work are 1) the models may not be relevant to the population of interest and 2) the cutoffs used to define a biomarker-high population will be different to cutoffs you’d use for an assay in the clinic. To manage these challenges, you need a good deal of data to help define where best to set a cutoff that aligns with patient benefit, and iterate as new data is made available. In working with Tempus’ RNA and real-world patient data, we can get a molecular view of what the tumor will look like, and then use analytical tools to select the right patients. This approach not only speeds research and development, but also helps manufacturers maximize their returns on investments by considering both real-world and clinical-trial generated data much more dynamically than has historically been possible. |
Mike: How does RWD inform commercialization and companion diagnostic development?
|
|
Dr. Kumar: We use RWE to understand a range of topics, for example, to decide in which space we want to develop our product. We can use data to validate biological hypotheses and assess whether we have the right data to test our theories. We also use RWE to help define the standard of care. With RWD on patient treatment history and outcomes, we can better understand the competition, then prioritize our work accordingly. |
|
Dr. Lin: Data is a powerful tool for both patients and manufacturers. We can analyze the trajectories of thousands of patients, detect the presence of a biomarker that a given treatment will target, then simulate how the treatment will impact an individual to a high degree of statistical power. That’s extraordinarily powerful, and because cancer is so heterogenous, it only emerges when you have generated a massive data set. Today, a majority of all approved drugs in oncology have wide indications, but in the next few decades that balance will flip toward very effective drugs for a much smaller set of patients. This is the promise of precision medicine – bringing the right drugs to the right patients. Our cyclin dependent kinase 9 (CDK9) inhibitor is one example of a product with a small set of patients whose cancers depend on CDK9-driven oncogenic transcription. This is where the combination of empirical data – generated in translational systems using clinical-grade measurements – and the power of the Tempus platform will be transformative, for example, in developing companion diagnostic tools to identify patients who will benefit most from the treatment. |
Mike: How is your approach to heterogeneous data sources impacting development timelines?
|
|
Dr. Kumar: Genomic data has allowed us to stick to our timelines, and even accelerate them, by helping us prioritize which indications to target. Instead of spending up to eight months in wet lab biology, we used RWD to — within one month — narrow our focus to one indication. This has been hugely beneficial in terms of time and cost savings. We can also more meaningfully define our patient selection strategy for trial development, which will increase our probability of success. If we can enroll a more targeted group of patients who may actually benefit from the treatment, we can make clinical trials smaller and smarter. Not only does this reduce the burden on patients, but from a bottom-line perspective, it’s how to perform a trial as quickly and safely as possible. |
Mike: What advice would you give other biopharma organizations looking to incorporate genomic data?
|
|
Dr. Kumar: First, select the right data and analytic partner whose datasets and people can help you get the answers you need. |
|
Dr. Lin: It’s important both to be willing to ask the hard and right questions of the data, and to have access to the data that allows you to ask those questions. By doing these two things, you position yourself to innovate. In our collaboration with Tempus, it’s been great to see both sides asking new questions of the data with guidance from the patients. This is key; the base premise for the analysis has to be right. More data ought to be more powerful, so there’s a huge responsibility to interpret and act on the data without getting clouded by its complexity. It’s critical to maintain focus on what’s most meaningful to patients both ahead of and as part of diagnostic and commercial development. |
Learn more

Learn more
Leveraging multimodal RWD in companion diagnostic development
Perspectives from biotech and biopharma leaders
DOWNLOAD GUIDEStay informed
Be notified whenever Tempus publishes new and relevant research, webinars, and other resources.
Sign up-
12/11/2025
Driving enterprise value with RWD
Hear from biotech CEOs and VC leaders as they discuss how real-world data can inform strategic decision-making in biotech companies.
Watch replay -
11/11/2025
A new era of biopharma R&D: The TechBio revolution—realities and the next frontier
Join Tempus and Recursion leaders to explore their strategic TechBio partnership. Learn how they use AI and supercomputing with petabytes of data to accelerate drug discovery and development. See the impact on biopharma R&D's future.
Watch replay -
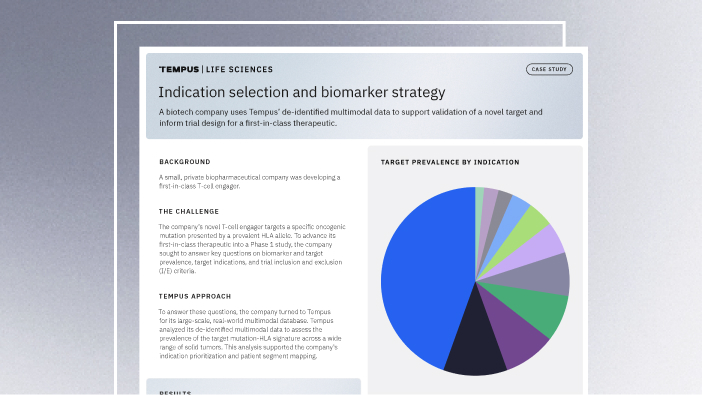
11/14/2025
Validating a novel target and informing trial design for a first-in-class therapeutic
Discover how a biopharma company used Tempus’ de-identified multimodal data to support validation of a novel target and inform trial design for a first-in-class therapeutic.
Read more