-
PROVIDERS
REGISTER NOW
Navigating new frontiers in breast cancer care pathway intelligence: The role of providers and AI
Tuesday, July 29th
2:00pm PT, 4:00pm CT, 5:00pm ET -
LIFE SCIENCES
REGISTER NOW
Closing Care Gaps with AI: The Next Competitive Edge in Pharma
Monday, July 14
9am PT, 11am CT, 12pm ET -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
Clinical Trial Enrollment
Tempus TIME
TIME gives you access to a network of providers and patients to accelerate enrollment in your clinical trials.
Contact us-
UPCOMING WEBINAR:
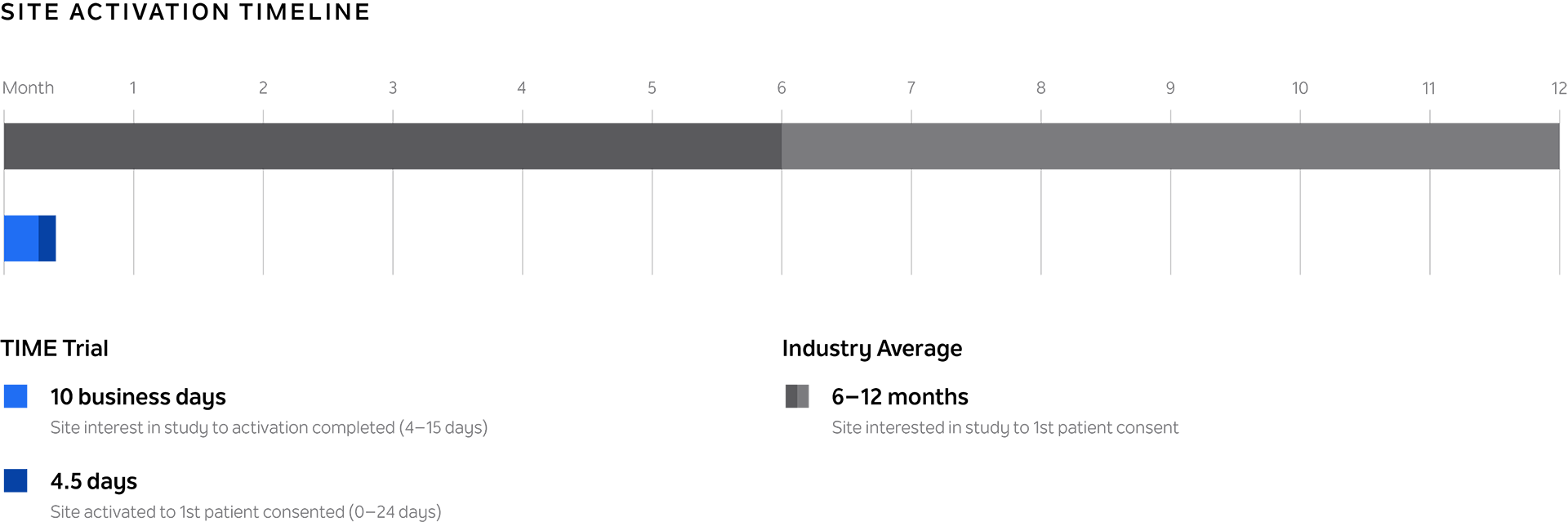
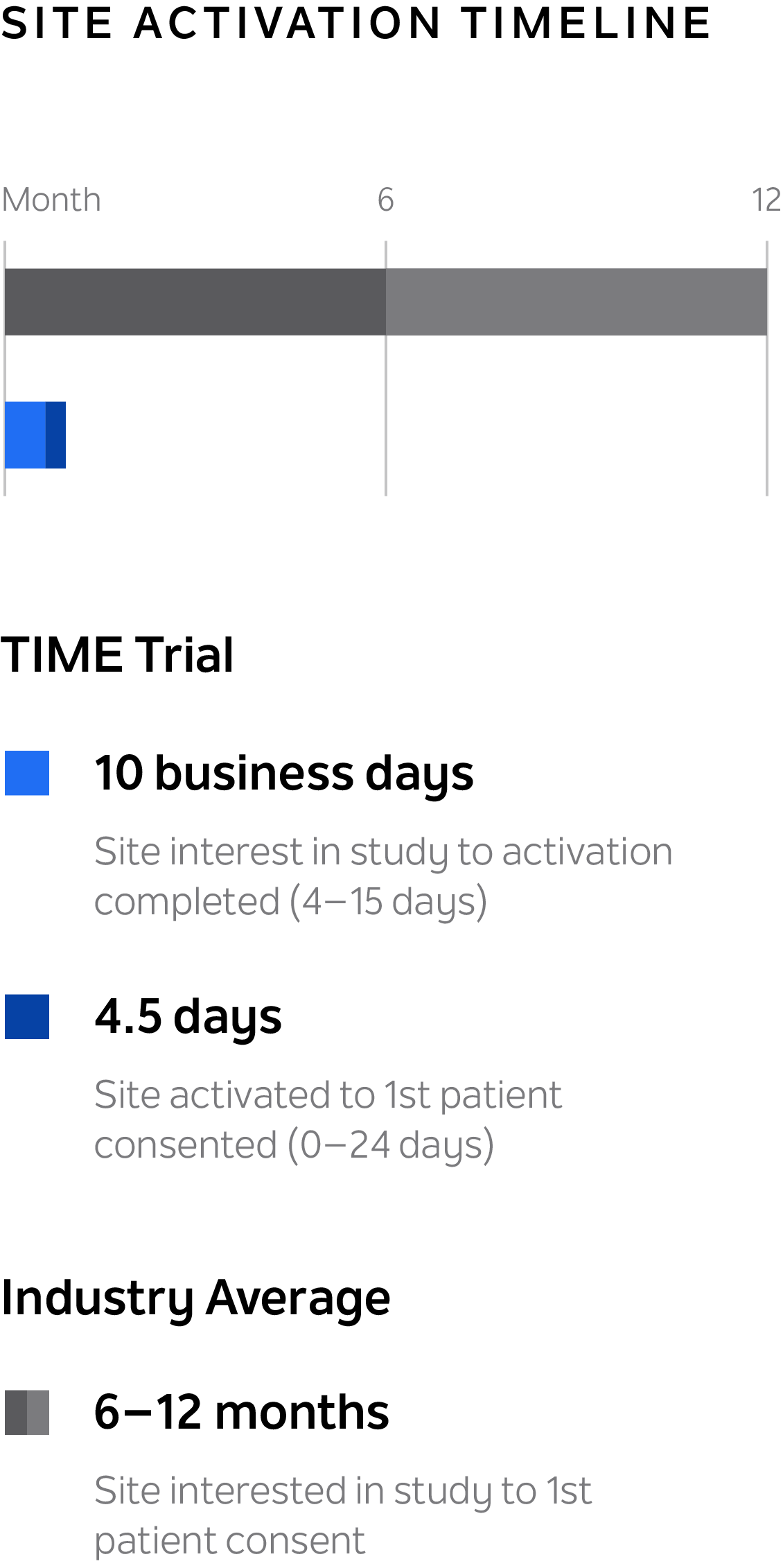
Reduce trial site startup time
Open trial sites consistently and reliably in ~10 business days for just-in-time trial activation, compared to the industry average of 8 months.
Read case study -
UPCOMING WEBINAR:
Find the right patients
Identify eligible patients with the highest likelihood of enrolling in your trial with our enhanced trial matching services — a unique combination of software, machine learning, and clinical experts.
Read case study -
UPCOMING WEBINAR:
Increase productivity of your sites
Leverage our pre-qualification process and team of experienced physicians, nurses, and research coordinators to get the most out of your participation in TIME.
Read case study
The TIME Program
The TIME program complements your traditional trial sites by bringing your study to patients faster, in locations where they already receive care. We do this through enhanced patient matching, rapid site activation, and site engagement initiatives.
A pre-qualified site is activated just-in-time when a subject is ready to join your study. Through a series of streamlined activities and agreements, TIME can help you activate new sites for your trial in ~10 business days for just-in-time trial activation. Our vast TIME network of academic medical centers and community-based sites continues to grow, and enables trial access for all patients — regardless of location.
Reimagine clinical trials
Tempus combines data and artificial intelligence to reimagine clinical trials across study design, site selection, patient enrollment, CRO capabilities, and post approval.
Just the numbers
-
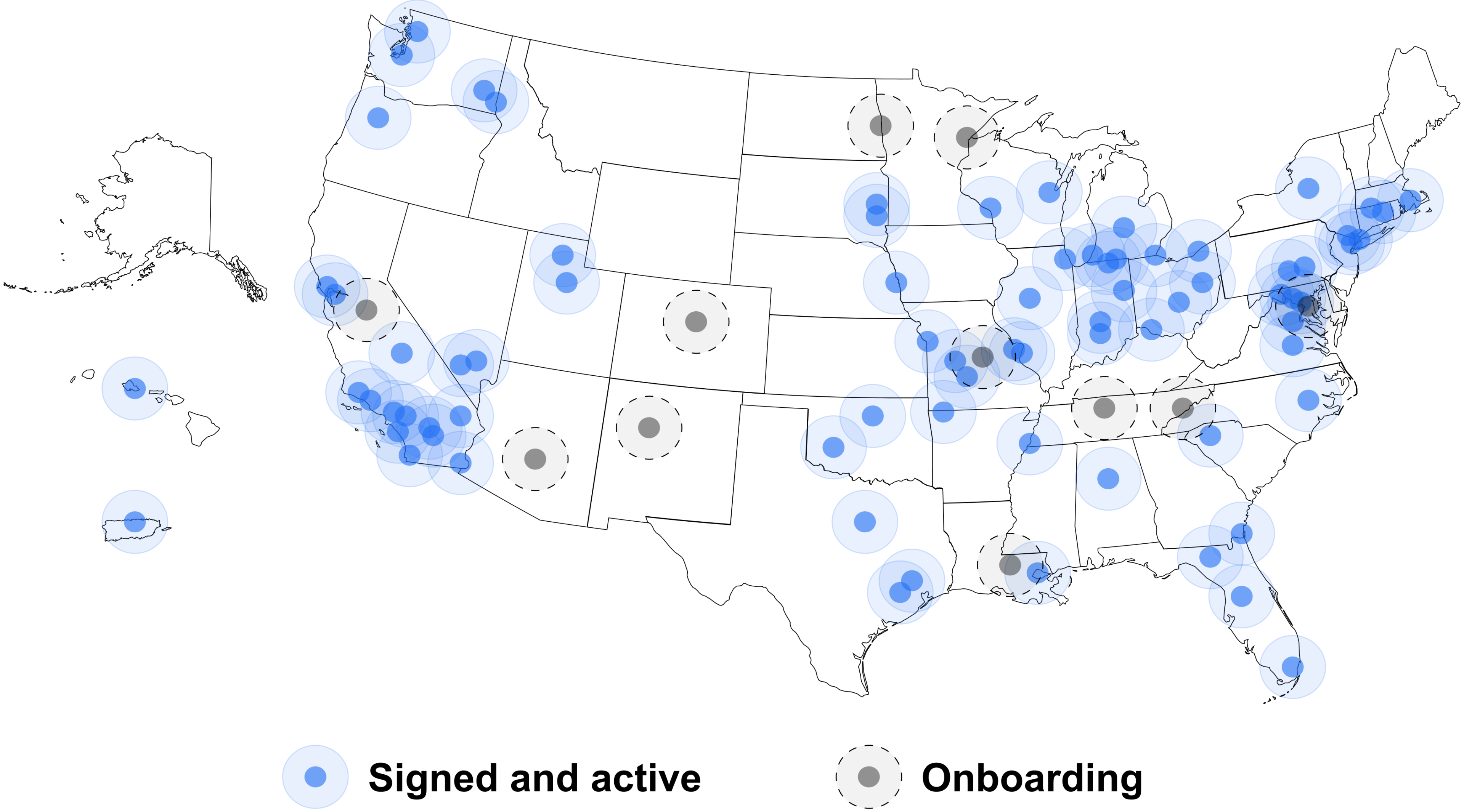
~100
sites representing 800+ clinical locations
-
1.6M
cancer patients covered by the TIME network
-
250+
clinical trials have been active
-
30K+
patients have been identified for potential enrollment in clinical trials in our network
-
~10
business days for just-in-time trial activation
Enhanced patient matching
TIME uses a comprehensive approach to identify patients who are eligible for your trial.
- Our flexible rules framework allows for precise structuring of your inclusion/exclusion criteria to programmatically identify patients in your target cohort(s).
- Comprehensive EMR connections and integrated machine-learning models enable evaluation of patients based on both clinical data and molecular data — regardless of the sequencing provider. This includes data elements across several categories, such as diagnosis, tumor/normal DNA sequencing/molecular pathology, treatments, and outcome, as well as immunohistochemistry (IHC) imaging, whole-exome RNA sequencing, and emerging immuno-oncology (IO) data from DNA/RNA.
- All potentially eligible patients are reviewed by a Tempus Nurse Navigator to assess eligibility and determine proper timing of communication with the physician and research team.
- Site-facing web portal enables providers and research teams to easily track patients and facilitate enrollment.
- Patients who meet match criteria are identified for further follow-up by their care team.
Tempus Link
Identify potential clinical trial patients, streamline trial match workflows, and accelerate enrollment—all within one AI-powered platform.
Clearly defined inclusion/exclusion criteria and curated patient data allow Tempus Nurse Navigators to precisely assess patient eligibility within a single tool. Our checklist user interface provides transparency into the trial matching logic and guides Nurse Navigators through a secondary review.
Data shown is not real patient data.
Learn more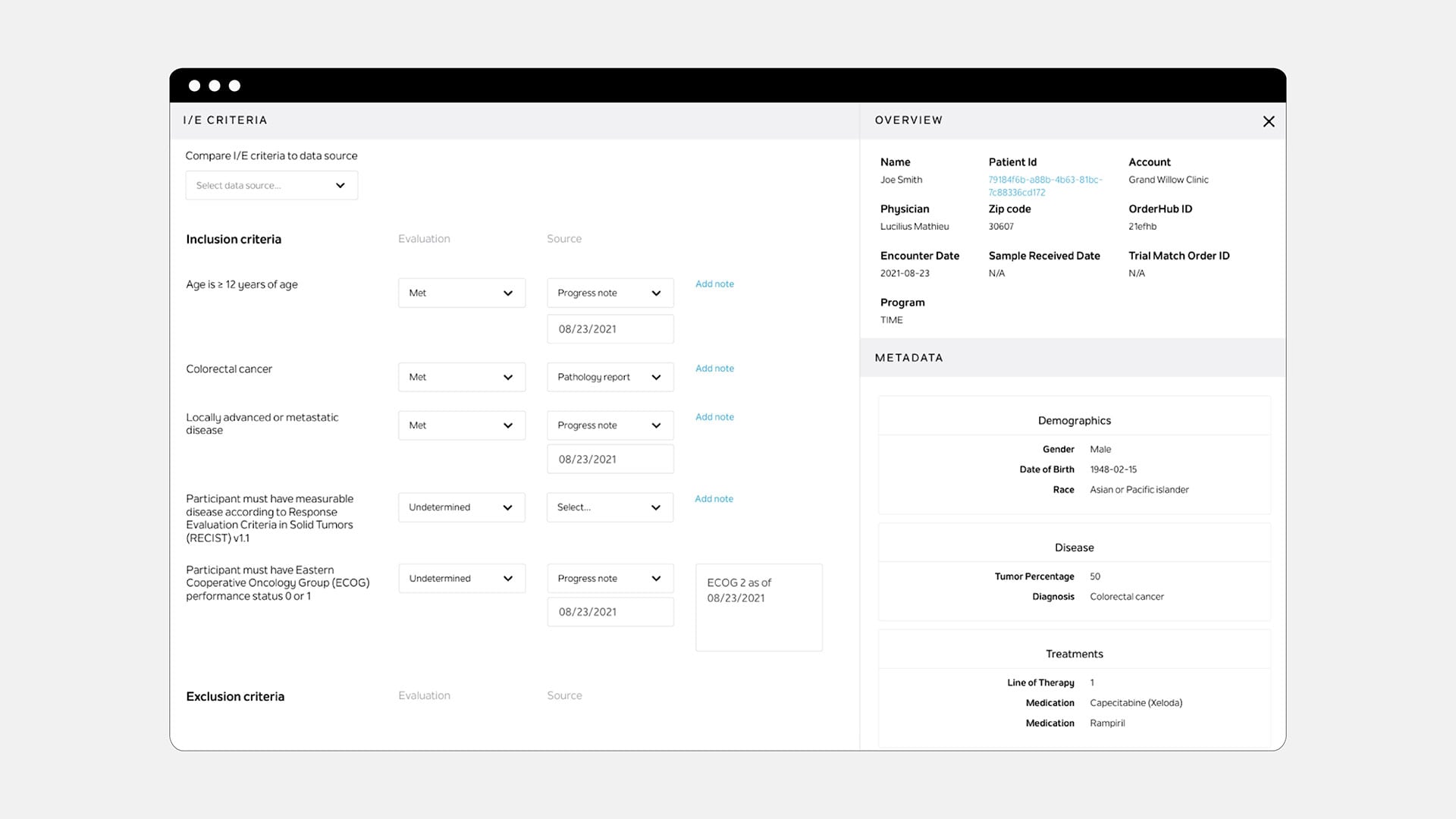
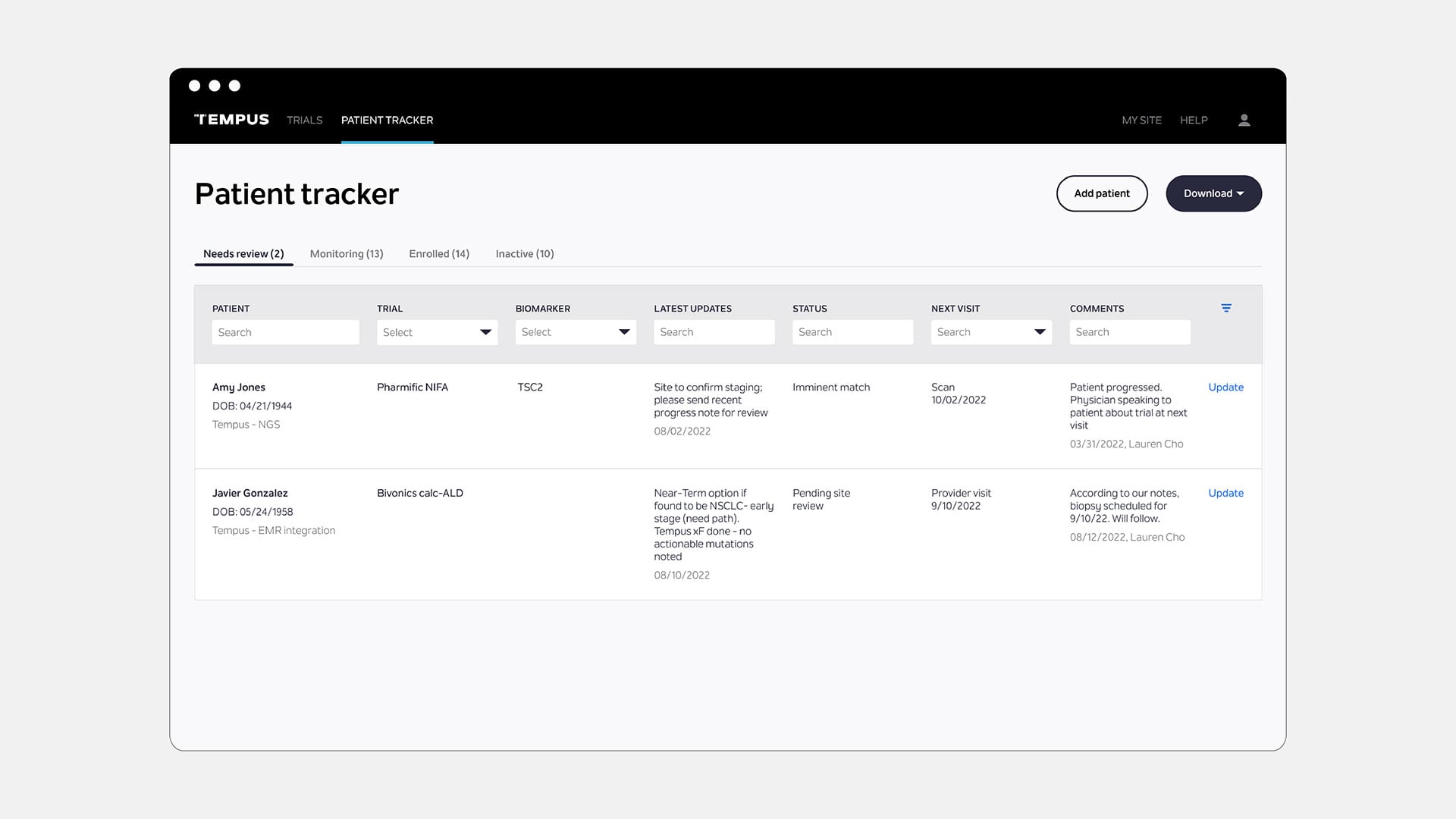
Tempus Patient Watchlist Tracker for Sites
Our patient tracker makes it easy for TIME trial sites to monitor patients and collaborate with Tempus throughout the screening and pre-enrollment process.
Data shown is not real patient data.
Rapid site activation
TIME sites are consistently activated in ~10 business days for just-in-time trial activation. Research standardization and on-site support enables operational efficiency to make this possible.
- Pre-qualification process to assess site’s research capabilities
- Rate card with pre-negotiated costs for over 300 budget items that inform trial budget
- Clinical trial agreement for all sites, eliminating repetitive, prolonged negotiations
- Informed Consent Form and single, central Institutional Review Board via Advarra to streamline regulatory submissions
Site engagement initiatives
TIME increases awareness of your study through ongoing clinical outreach with clinical trial sites.
- Trial matching tools enable sites to efficiently self-identify potential subjects and request additional pre-screening by a Tempus Nurse Navigator.
- Nurse Navigators follow up on watchlists and collaborate closely with sites to ensure timely next steps for patients.
- Electronic notifications are sent to treating physicians and Principal Investigators when potential subjects are identified.
- Experienced physicians and nurses work directly with TIME sites to evaluate new studies, determine patient eligibility, and discuss patient treatment options.
- Select sites are paired with a dedicated, on-site Tempus Clinical Research Coordinator to further embed TIME into a site’s research processes.
Trusted by hundreds of biopharma to power drug development
The information on this page is intended for life sciences companies and focuses on research and development applications.