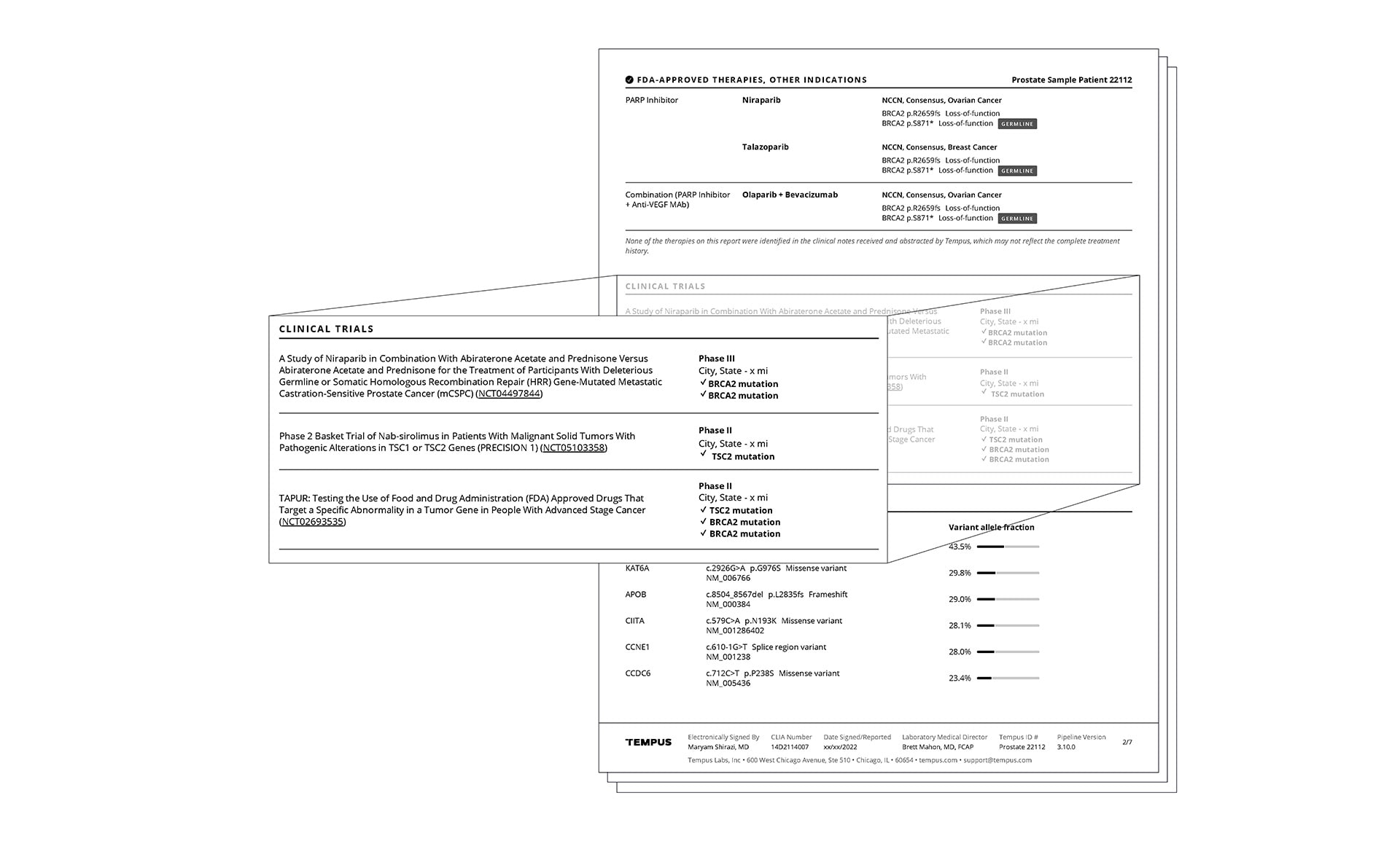
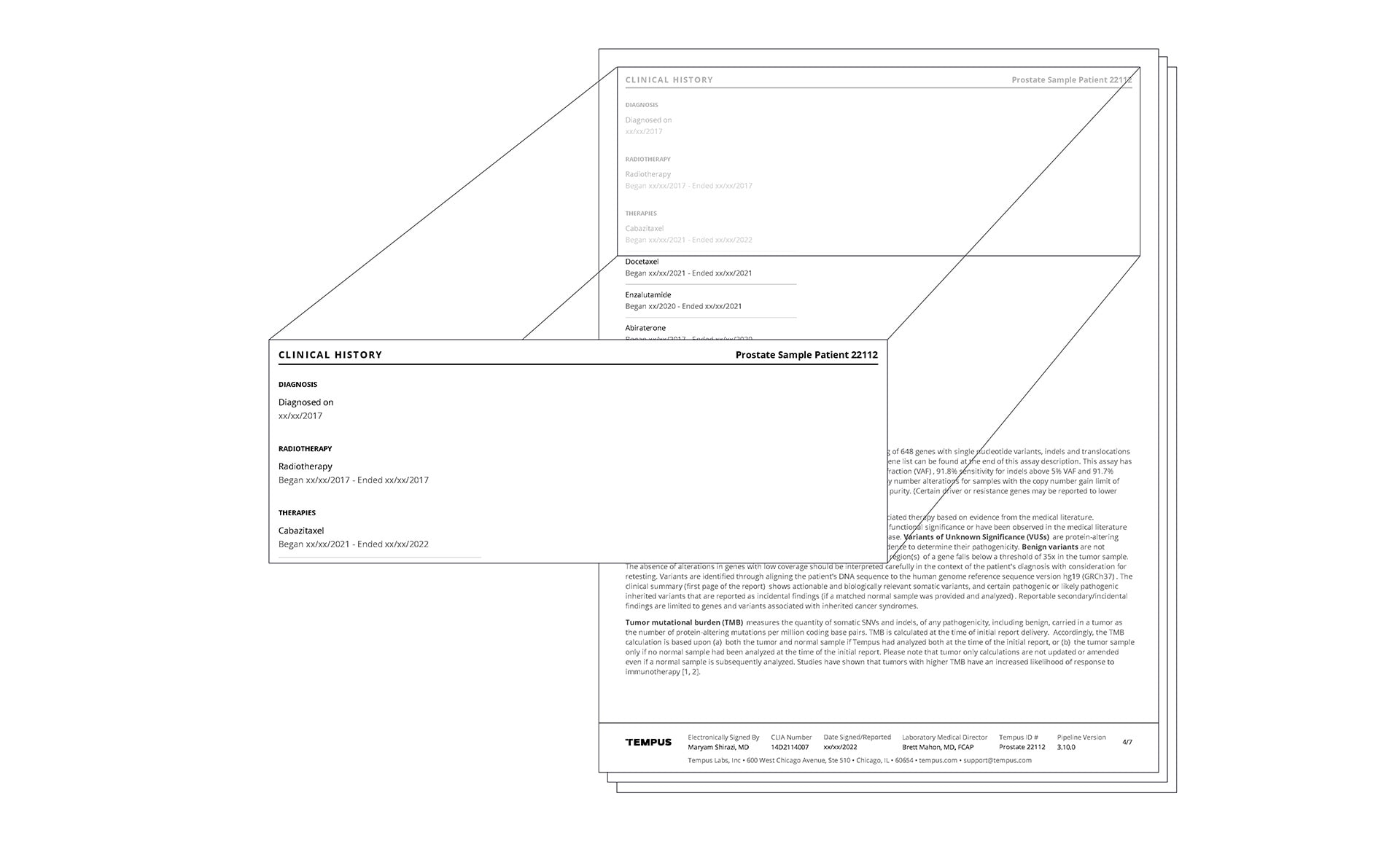
Actionable molecular findings.
-
PROVIDERS
Read more
Browse our latest selection of abstracts, manuscripts, and presentations.
- LIFE SCIENCES
- PATIENTS
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.