-
PROVIDERS
REGISTER NOW
Navigating new frontiers in breast cancer care pathway intelligence: The role of providers and AI
Tuesday, July 29th
2:00pm PT, 4:00pm CT, 5:00pm ET -
LIFE SCIENCES
REGISTER NOW
Closing Care Gaps with AI: The Next Competitive Edge in Pharma
Monday, July 14
9am PT, 11am CT, 12pm ET -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
Tempus xF+ Liquid Biopsy Assay
Tempus is proud to offer one of the largest available liquid biopsy (LBx) panels on the global market. The panel interrogates 523 genes focused on oncogenic and resistance mutations in cell-free DNA (cfDNA) to help identify therapy opportunities.
TEMPUS xF+
-
Depth of Sequencing (Avg.)
DNA sequencing is performed to >5,000x and >1,500x unique coverage for enhanced and additional regions, respectively.
-
Platforms Used
Illumina NovaSeq
-
Accepted Sample Types
Peripheral blood
-
Alterations Identified
SNV, insertions / deletions, CNVs, gene rearrangements from select genes, MSI, bTMB
-
Sensitivity
98.3% for SNVs (≥ 0.2% VAF), 95.5% for indels (≥ 0.25% VAF), >99.9% for CNGs, 96.8% for rearrangements, 90% for MSI-H, 78.5% for bTMB1
-
Gene List Download xF+ Validation
enhanced testing
Discover actionable alterations with complementary testing
Non-overlapping actionable gene alterations may be discovered using complementary tissue and liquid biopsies. In one study of NSCLC patients, the addition of plasma sequencing increased targetable mutation detection to 35.8% compared to 20.5% with tissue alone.2
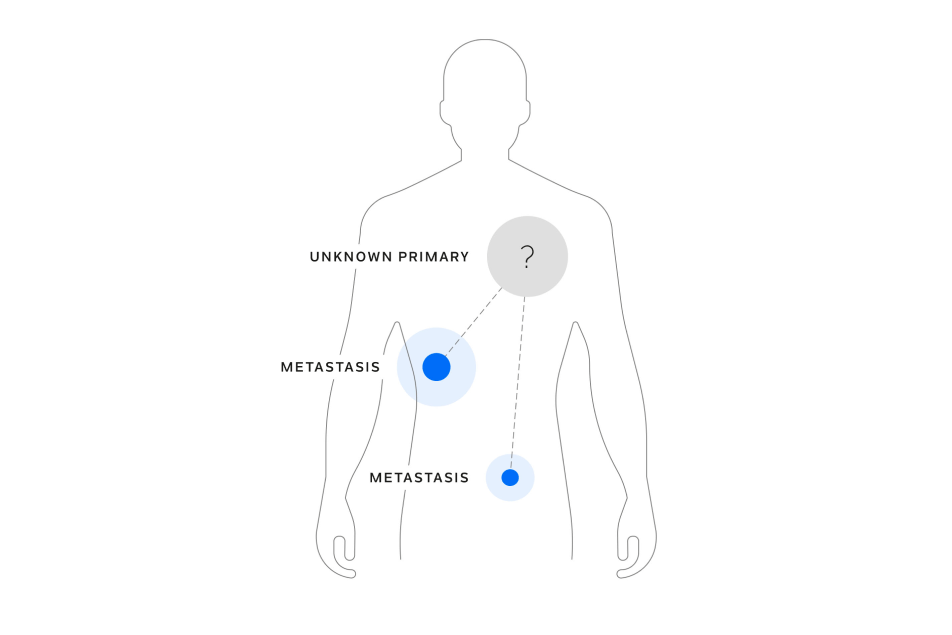
EVOLUTIONARY INSIGHT
Monitor disease recurrence after treatment
Longitudinal testing can help in the identification of genomic alterations predictive of response and resistance to targeted therapies and evaluation of genomic heterogeneity.
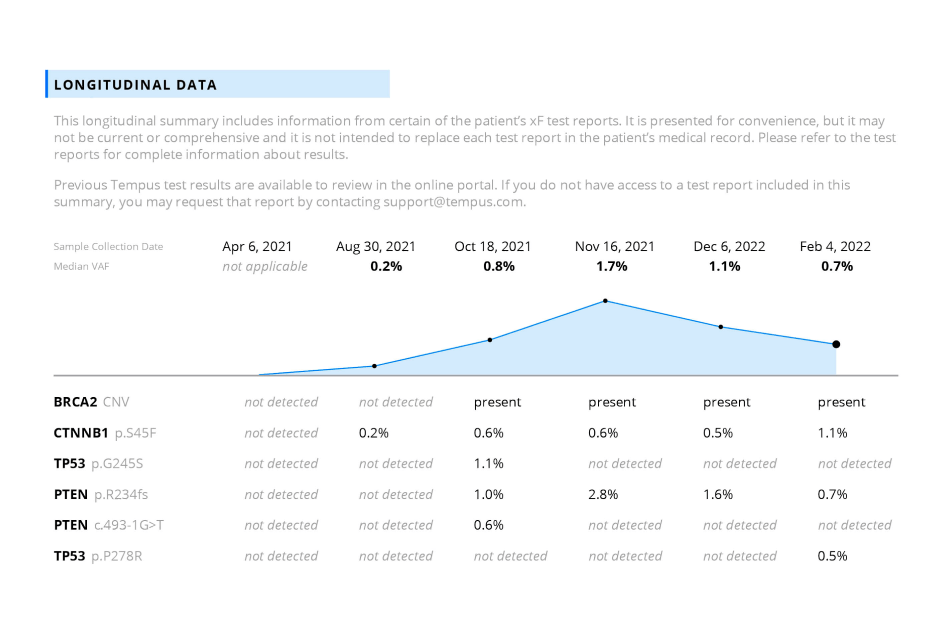
- Tempus xF+ validation paper.
- In a subset of 229 NSCLC patients, tissue testing alone detected targetable mutations for 47 patients, whereas plasma sequencing increased targetable mutation detection to 82. Aggarwal C et al. Clinical Implications of Plasma-Based Genotyping With the Delivery of Personalized Therapy in Metastatic Non–Small Cell Lung Cancer. JAMA Oncol. 2019;5(2):173–180. doi:10.1001/jamaoncol.2018.4305
Trusted by hundreds of biopharma to power drug development
The information on this page is intended for life sciences companies and focuses on research and development applications.
Interested in learning more about our liquid biopsy offering?
Contact us today.