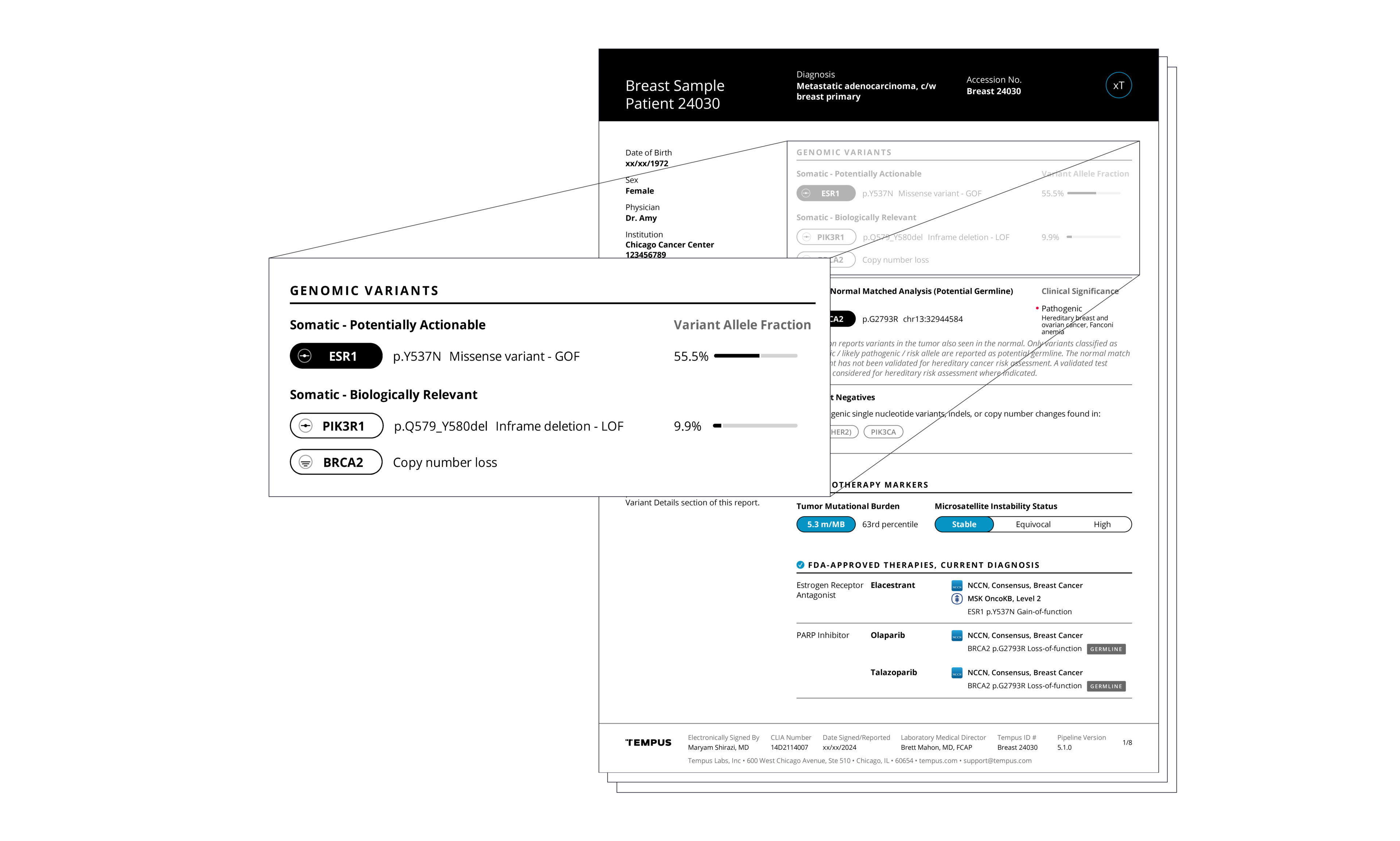
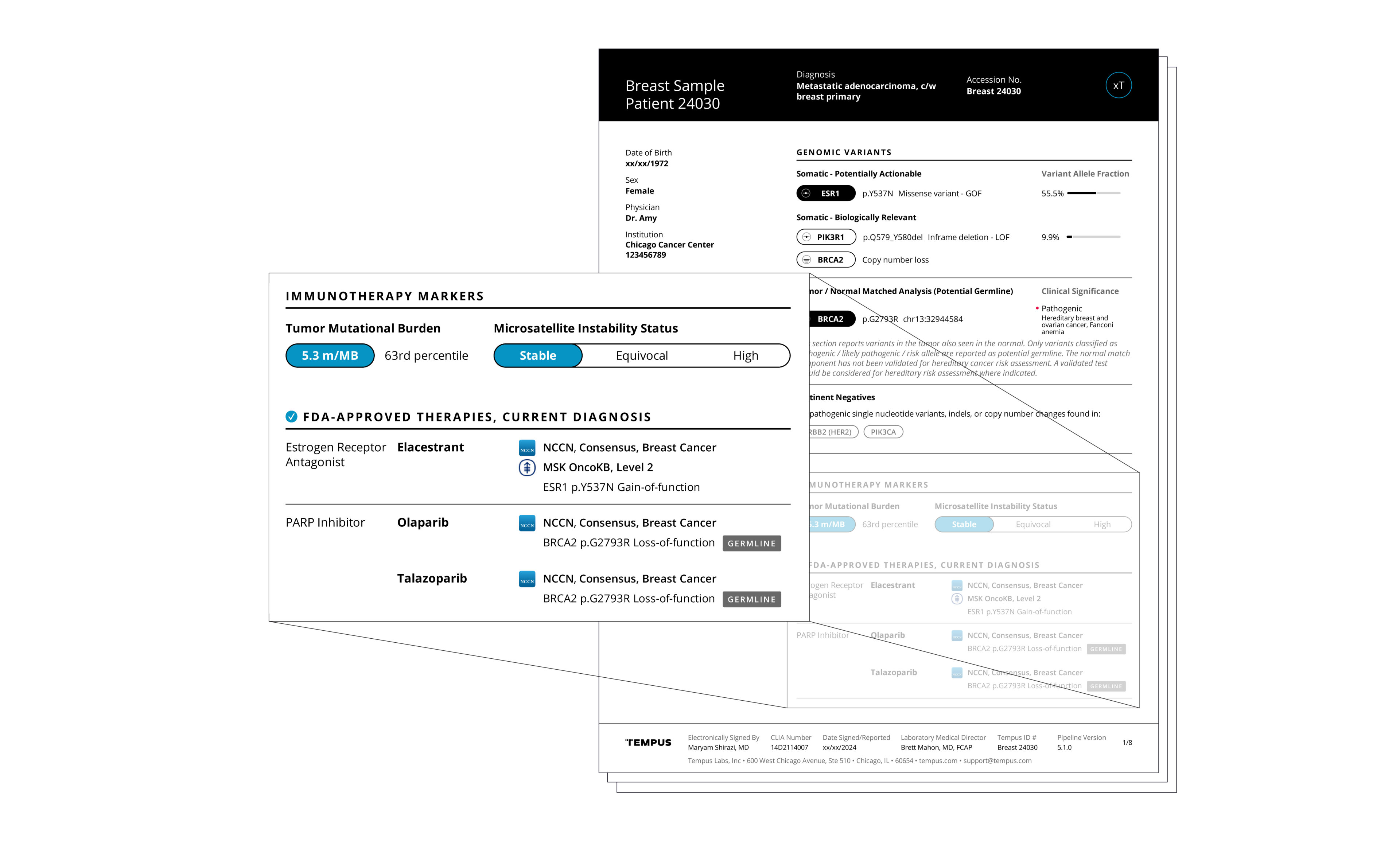
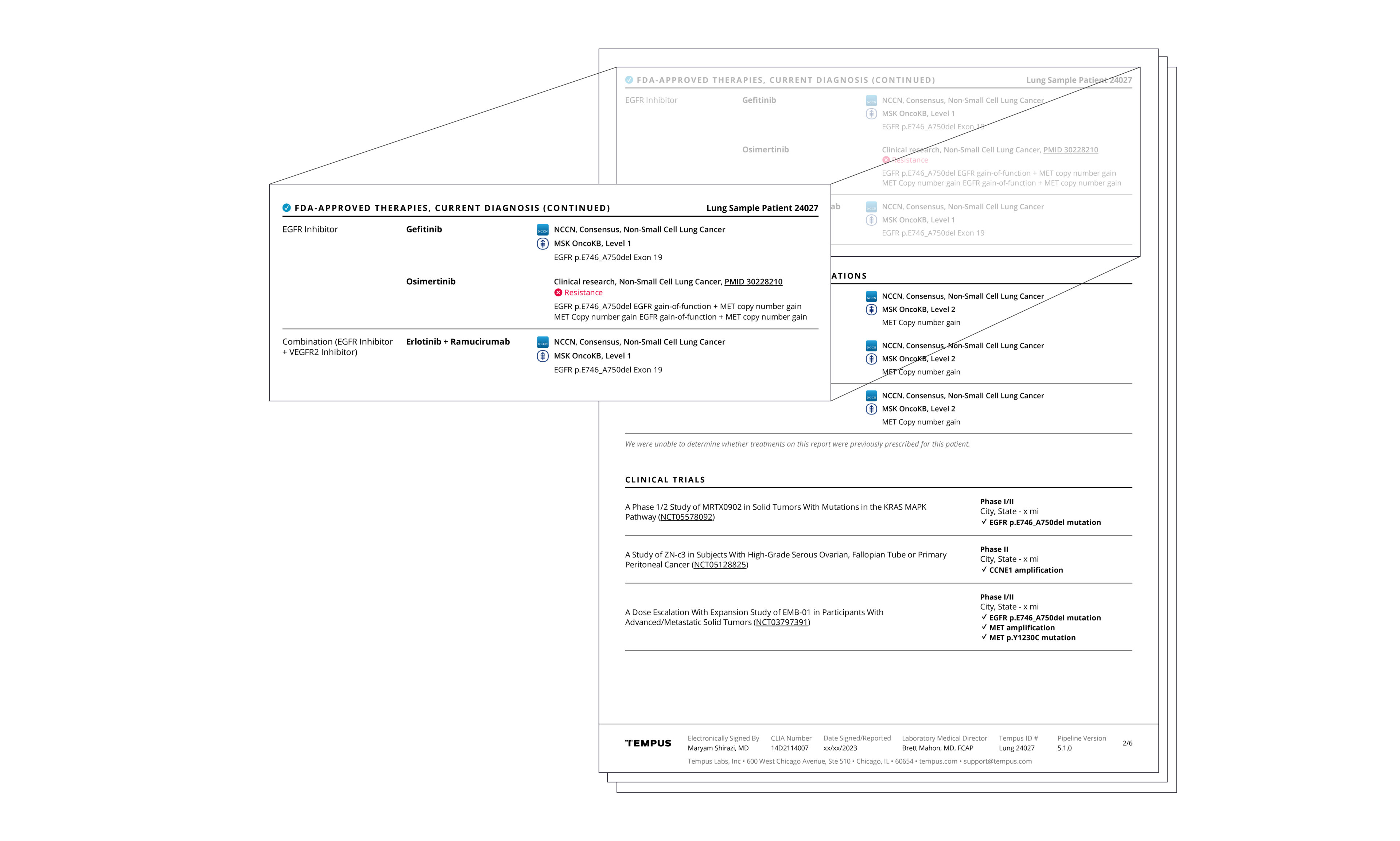
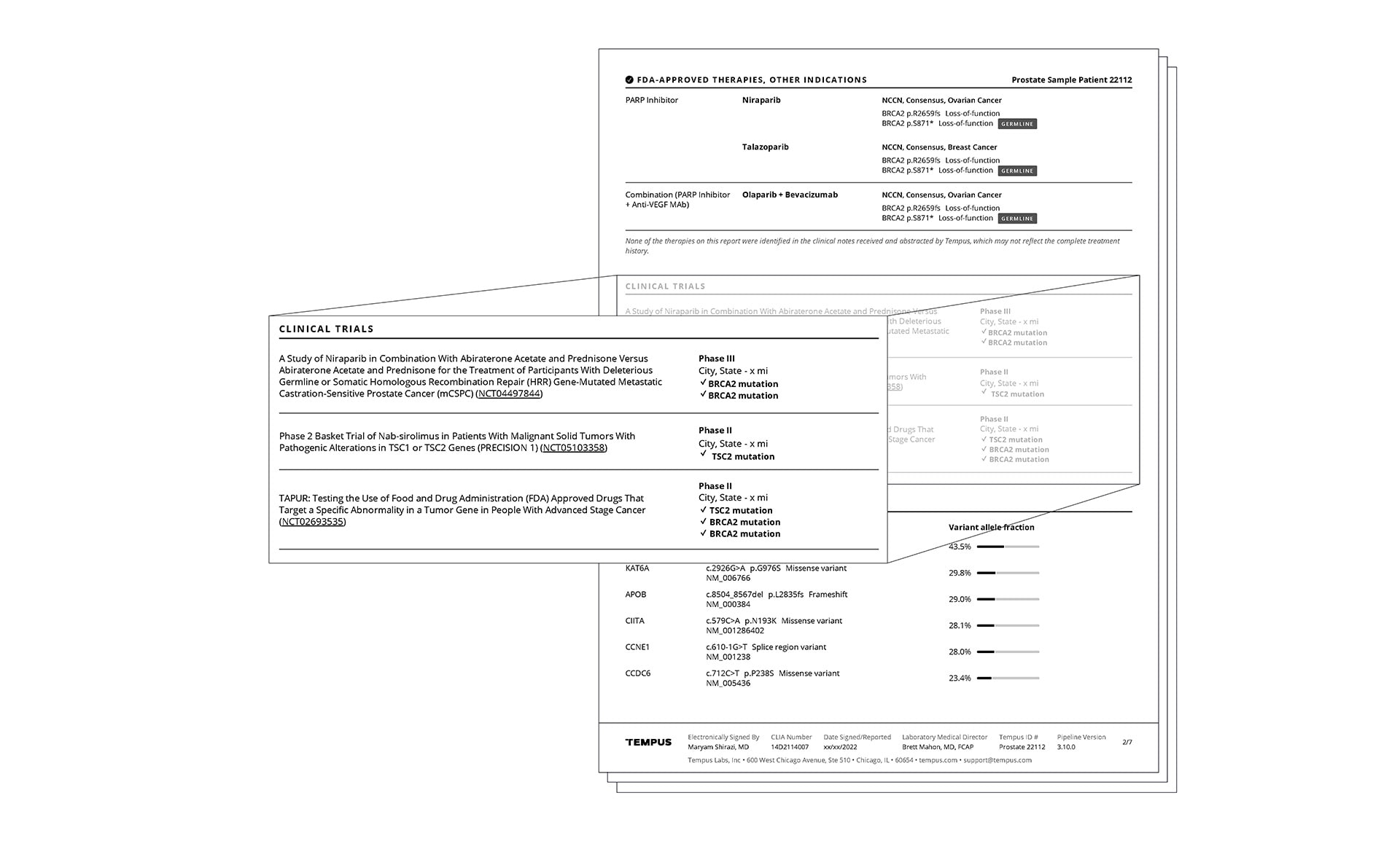
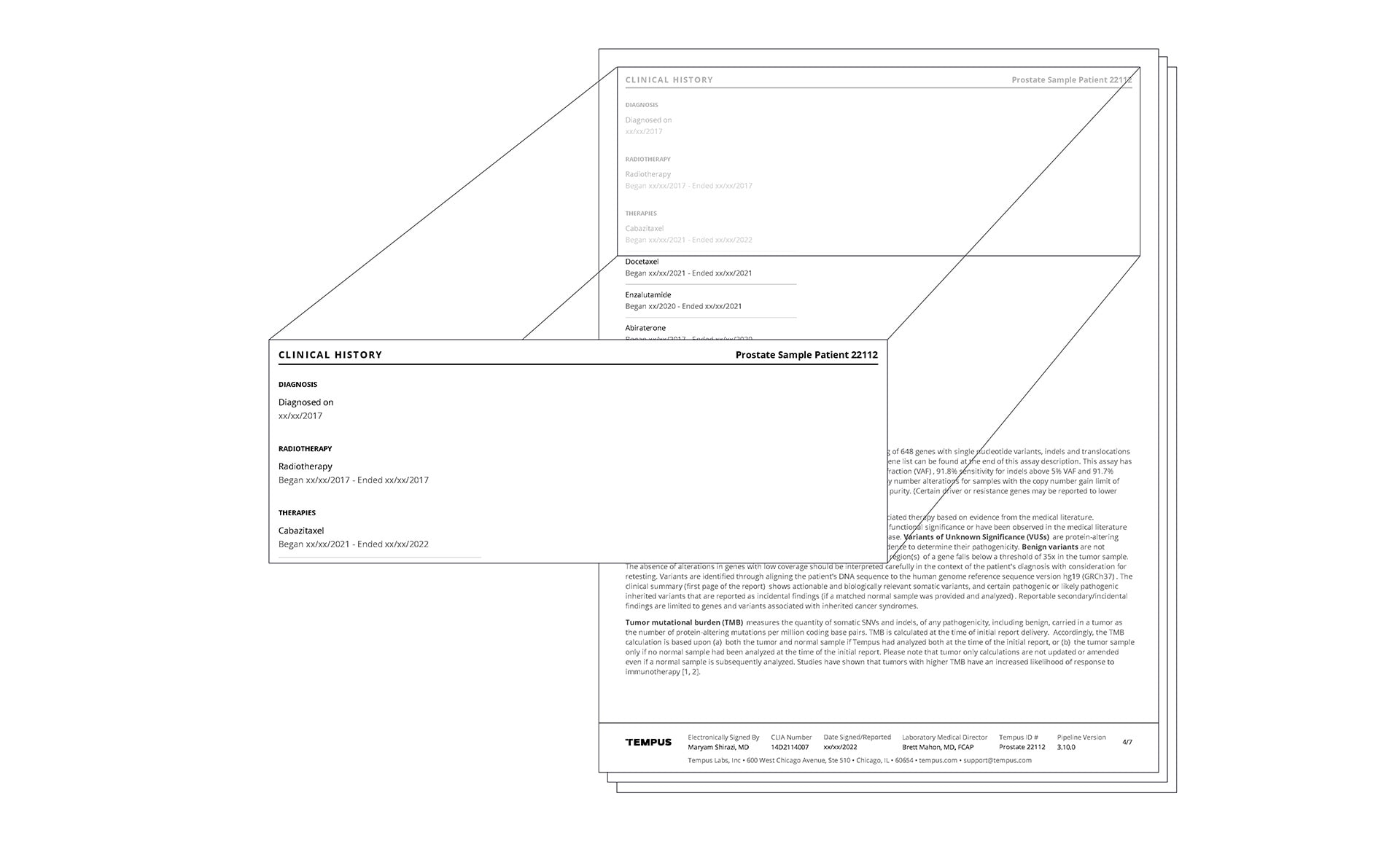
Actionable molecular findings.
-
PROVIDERS
New MRD Medicare Coverage for Select Indications*
*When coverage criteria are met. Additional criteria and exceptions for coverage may apply.
-
LIFE SCIENCES
Learn more
Building the engine to scale data abstraction through AI
Learn how Tempus’ AI engine transforms unstructured clinical text into analysis-ready real-world data -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS