-
PROVIDERS
Register now
Are you getting the full picture? A webinar series on the power of comprehensive intelligent diagnostics
-
LIFE SCIENCES
REGISTER NOW
The RNA advantage: A multimodal approach to accelerating oncology R&DThursday, May 22, 2025
10 AM PT / 12 PM CT /
1 PM ET -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
05/09/2025
Q&A: Integrating MRD and IO Monitoring with the Tempus portfolio: An oncologist’s perspective
This Q&A article features a discussion between Dr. Allan Espinosa of Arizona Oncology and Dr. Ezra Cohen, Chief Medical Officer at Tempus, exploring the practical application of Tempus’ tumor-naive and tumor-informed MRD and IO treatment monitoring portfolio in clinical practice.
Speakers
Ezra Cohen, MD
Chief Medical Officer of Oncology, Tempus

Allan Espinosa, MD
Arizona Oncology

Chief Medical Officer of Oncology, Tempus

Allan Espinosa, MD
Arizona Oncology

In the rapidly evolving landscape of oncology, Tempus offers new avenues for enhancing patient care. A critical aspect of this advancement lies in our ability to leverage testing to detect minimal residual disease (MRD) and monitor treatment response to immunotherapy (IO).
Selecting MRD and IO monitoring strategies across the patient journey
Tempus offers a few different testing approaches—can you explain the difference between tumor-naive and tumor-informed testing and when you might use each? |
Dr. Espinosa: Tumor-informed testing offers high specificity and sensitivity because it’s tailored to a patient’s unique tumor profile. While it traditionally had a longer turnaround time, it’s invaluable for long-term surveillance and monitoring after checkpoint inhibitor therapy. Once a tumor-informed profile is established, subsequent tests are much quicker. Tumor-naive testing, on the other hand, provides faster results, making it ideal for situations demanding quick decisions, such as in high-risk early-stage colon cancer to guide adjuvant chemotherapy timing. It’s also beneficial when tissue samples are limited. |
What key factors influence your choice of MRD or IO treatment response monitoring? |
Dr. Espinosa: The primary factor is the clinical scenario. In the adjuvant setting (e.g., after surgery for stage III lung cancer or melanoma), IO treatment response monitoring early in therapy can help predict a patient’s response even before imaging. In the metastatic setting, especially with checkpoint inhibitors, it’s also very helpful. For ongoing surveillance, I prefer tumor-informed MRD testing, as clinical trials have shown its ability to detect recurrence earlier than imaging alone. I typically use it every three to four months for patients under active surveillance. |
Integrating MRD results into clinical decision-making
How do you integrate MRD results (positive, negative, or dynamic changes over time) into your clinical decision-making? |
Dr. Espinosa: It’s highly dependent on the tumor type, with colon cancer having the most validated data. A negative MRD result is reassuring, but I always correlate it with clinical and imaging findings. A positive result prompts me to monitor more closely and often repeat testing to observe trends. A consistent rise in MRD levels often leads me to consider earlier therapeutic intervention or clinical trial enrollment, even before radiological progression. I emphasize to patients that MRD is an evolving technology but a growing asset in our surveillance toolkit. |
How do you communicate MRD results to your patients, and are they becoming more aware of this testing? |
Dr. Espinosa: Some patients are becoming aware, especially through community outreach or advocacy. When explaining results, I stress that MRD testing is promising but still being validated across different cancers. I present it as a tool alongside imaging and clinical judgment. For negative results, I explain it’s reassuring but not a definitive guarantee. For positive results, I indicate the potential presence of microscopic disease and discuss next steps, often involving repeat testing to confirm trends before significant treatment changes.
|
Clinical utility of IO treatment response monitoring
Can you share an example of how IO treatment response monitoring has influenced your management decisions? |
Dr. Espinosa: I’ve primarily used it to pause treatments. For example, in several stage IV lung cancer patients on checkpoint inhibitors for nearly two years who developed toxicities—liver issues, kidney problems, or significant skin toxicities like pruritus. When the IO treatment response monitoring shows a negative result, I feel much more confident pausing therapy, knowing molecularly the disease appears controlled. In melanoma, if I see a rising trend in ctDNA after two to three tests, even without clear radiologic progression, I start thinking about switching therapy—especially in BRAF-positive patients where we have options like MAP kinase inhibitors. |
How do you interpret discordant results where imaging suggests progression but ctDNA is decreasing? |
Dr. Espinosa: This is where treatment response monitoring is invaluable. Pseudoprogression is a known phenomenon with immunotherapy. If imaging shows worsening but the patient feels clinically well and ctDNA is decreasing, I use that as evidence to reassure patients that it’s likely pseudoprogression, not true progression. I explain that ctDNA has a very short half-life—about two hours—so it’s a more immediate and reliable marker than imaging alone, which can show inflammation rather than tumor growth. |
What is your typical cadence for ordering IO treatment response monitoring and how often are you testing? |
Dr. Espinosa: I align it with treatment cycles. Depending on whether I’m using a four-week or six-week checkpoint inhibitor schedule, I order the ctDNA test with labs about two days before treatment. Sometimes results are back in time for the visit, and that real-time feedback is very reassuring for both me and the patient.
|
What has your experience been with the Tempus IO TRM offering, particularly regarding scheduling, logistics and turnaround time? |
Dr. Espinosa: It’s been excellent. Once the tumor signature is established, subsequent turnaround times are excellent, typically under a week. The ability to access results through the Tempus app on my phone is incredibly convenient — I can pull up results live during a patient visit and have the discussion immediately. |
In your experience, how has IO treatment response monitoring impacted your ability to predict long-term outcomes, like relapse-free survival or overall survival? |
Dr. Espinosa: It’s still early for me personally to assess long-term survival outcomes. However, what I have consistently observed is that ctDNA trends are predictive of early progression. If the ctDNA starts rising consistently over two or three measurements, it usually precedes clinical or radiographic progression. So it’s been very helpful for earlier intervention decisions, even if I don’t yet have mature survival data in my own patient cohort. |
Future landscape
How do you envision the role of MRD evolving in the next few years? |
Dr. Espinosa: I believe it will fundamentally reshape oncology practice. For cancers that reliably shed ctDNA, MRD will likely enable more precise treatment tailoring, potentially reducing overtreatment in the adjuvant setting and allowing for earlier intensification of therapy for low-volume disease. I anticipate it following a similar trajectory to Oncotype DX in breast cancer, where validated biomarkers guide treatment de-escalation. I’m optimistic that within 3-5 years, MRD will be integrated into standard guidelines for cancers like colon, melanoma, and lung.
|
What is the most critical investment you’d like to see for MRD and IO TRM testing in the next five years? |
Dr. Espinosa: For MRD, the priority is validation, validation, validation across all tumor types, with robust data on endpoints like progression-free and overall survival. For IO TRM, the focus should be on integration into standard clinical practice and payer acceptance, making it a routine tool to reduce over-reliance on imaging alone. |
| The integration of MRD and IO treatment response monitoring is advancing precision oncology by enabling tailored surveillance and earlier intervention. By applying tumor-informed and tumor-naive approaches based on clinical context, and using IO monitoring to assess efficacy before imaging changes may appear, clinicians can make informed management decisions, ultimately striving for more personalized strategies. Ongoing investment in these technologies will continue to drive improvements in patient outcomes and reshape cancer care. Explore Tempus’ xM portfolio here. Dr. Espinosa serves as a consultant to Tempus. |
-
04/02/2025
Development of a clinical algorithm to prognosticate response to immunotherapy
Discover how Tempus developed and deployed the Immune Profile Score (IPS)—a powerful algorithm that provides prognostic insights into patient outcomes following treatment with immune checkpoint inhibitors (ICIs)—in ~18 months. This case study highlights the AI-driven methodology, real-world validation, and the impact of IPS in precision oncology.
Read more -
03/25/2025
AI & ML in action: Unlocking RWD with GenAI through Tempus Lens
Discover how Tempus is equipping researchers with innovative AI solutions to fully leverage the potential of multimodal data. Gain insights from a panel of leaders across healthcare and life sciences as they discuss the impact of these advanced tools on delivering insights with speed.
Watch replay
Secure your recording now. -
03/11/2025
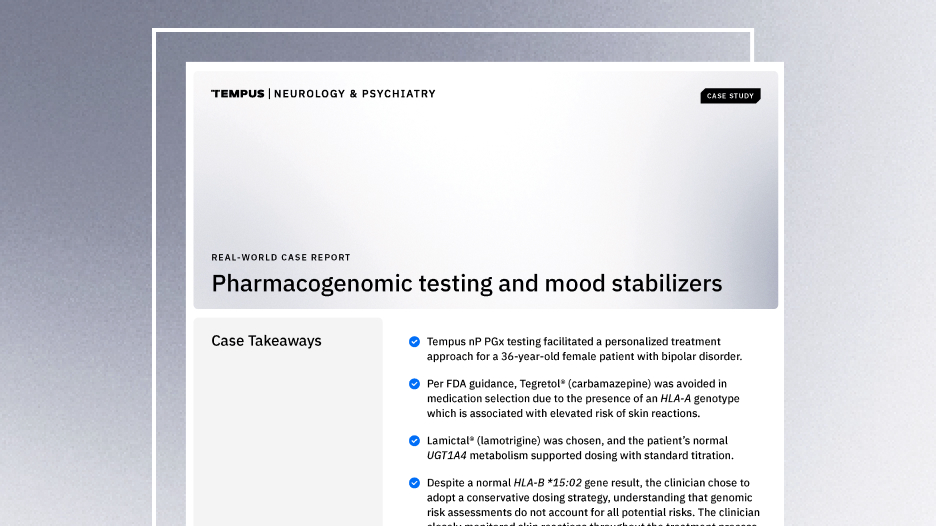
Case Report: Pharmacogenomic testing and mood stabilizers
This real-world case demonstrates how the Tempus nP pharmacogenomic test facilitated a personalized treatment approach for a patient with bipolar disorder.
Read more