-
PROVIDERS
Watch now
Are you getting the full picture? A webinar series on the power of comprehensive intelligent diagnostics
-
LIFE SCIENCES
ENROLL NOW
Tempus’ Patient-Derived Organoid ScreensEvaluate the efficacy of your preclinical compounds using fixed organoid panels designed for diverse therapeutic applications.Space is limited — enroll by June 30, 2025, to secure your spot.
-
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
Content
-
Case Studies
Using TIME to accelerate trial enrollment for Aadi Bioscience
The Tempus TIME program provided Aadi access to a network of providers and patients to accelerate enrollment for its PRECISION 1 TSC1/TSC2 phase 2 clinical trial. Read on to learn how the TIME program decreased time to enroll with the just-in-time activation of ~10 business days. -
Case Studies
Ultra-rapid site activation and subject consent in PARPi Study using Tempus TIME Program
The TIME program enabled activation of a new clinical trial site within 4 business days for a PARPi study. Read on to learn how the patient was able to dose within 10 days of being identified as a match for the trial. -
Case Studies
Sequencing for development and commercialization
If you’re wondering how Tempus’s sequencing services can help your project move forward, consider the experiences of these four organizations, who partnered with Tempus for prospective sequencing for clinical trials, retrospective sequencing and analyses, companion diagnostic development, and sponsored testing. -
Case Studies
Powering discovery: Tempus Multimodal Database
Targeting discovery is an important frontier in the fight against pancreatic cancer. Take a look at this example of how the Tempus Multimodal Database enabled a pharmaceutical company to more quickly identify pancreatic cancer subtypes’ molecular signatures. -
Case Studies
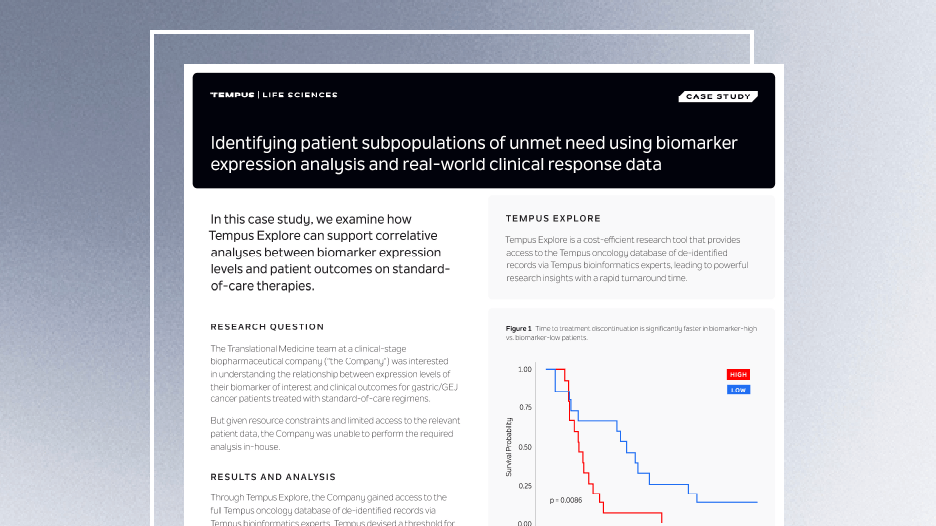
How to identify unmet medical need with Tempus RWD
See how Tempus RWD enabled a biopharma company to correlate biomarker expression levels to patient outcomes on SoC therapies. -
Case Studies
Enhanced biomarker discovery and design
Combining sequencing, analytics, and data licensing with Tempus allows you to uncover new insights across the research and development spectrum. This case study shows how one company made it happen.