-
PROVIDERS
REGISTER NOW
Navigating new frontiers in breast cancer care pathway intelligence: The role of providers and AI
Tuesday, July 29th
2:00pm PT, 4:00pm CT, 5:00pm ET -
LIFE SCIENCES
REGISTER NOW
Closing Care Gaps with AI: The Next Competitive Edge in Pharma
Monday, July 14
9am PT, 11am CT, 12pm ET -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
11/22/2022
Dual testing in metastatic breast cancer
Tempus research exploring the concordance of pathogenic variants across a variety of criteria for metastatic breast cancer patients.
Authors
Matt MacKay, PhD
Director, Molecular Analytics, Tempus

Director, Molecular Analytics, Tempus

Research summary
Tempus offers both a tissue based NGS assay (Tempus xT) as well as a cell-free DNA (cfDNA) NGS assay (Tempus xF), allowing clinicians flexibility in their diagnostic decisions. However, while each modality offers different benefits, concordance between the two types of tests is a source of ongoing study. To address this, Tempus researchers evaluated the concordance between Tempus xT and Tempus xF relative to cfDNA sampling frequency in a large, clinically annotated deidentified metastatic breast cancer (mBC) data set using the Tempus Lens platform. The team investigated the concordance of pathogenic variants across genes, time between tests, the variant allele frequencies, and mBC subtypes.
The study reviewed the records of 300 patients with stage IV mBC, 94% of which had at least one pathogenic alteration found between ctDNA biopsy and tissue NGS. When tissue and blood collection were ≤ 7 days apart (n=56), 77.8% of pathogenic tissue variants were found within each patient’s cfDNA, and 75.7% of pathogenic cfDNA variants were found within each patient’s tissue, showing high concordance of variant detection for each patient. At the individual variant level, 20% of pathogenic variants were uniquely detected by xF liquid biopsy, while 18% were detected solely in tissue by xT (fig.1). Notably, all ESR1 variants were more likely to be identified when analyzing cfDNA (cfDNA-only or concurrent) than tissue samples alone (fig. 2). Combined testing with xT and xF may provide a more complete picture of the patient’s genetic landscape than either testing method alone.
The concordance observed (62% concordance, ≤7 days) suggest that cfDNA NGS may reliably represent tissue genomics, but the increase in discordance over time also suggests a possible evolution of tumor genomics that can be measured by sequential testing with Tempus xF. Additionally, the detection of unique variants by each testing modality may indicate that using both tests could increase the overall variants detected compared to using either individually.
Next steps
Contact Tempus to discuss the findings with our team, and learn more about concurrent testing in breast cancer patients.
Unique variants may be detected between tissue samples and cfDNA
Figure 1
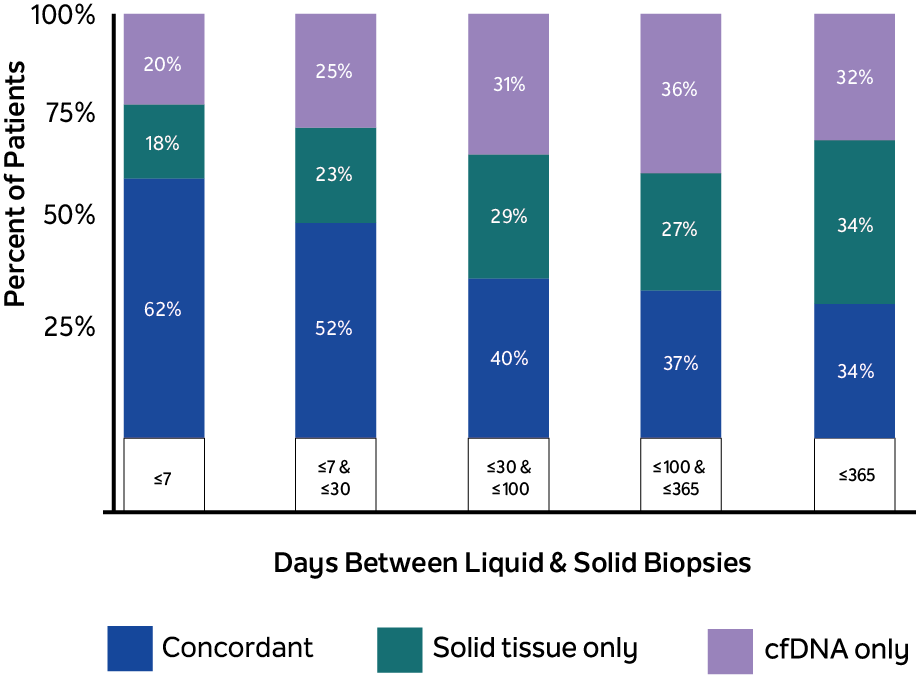
20% of pathogenic variants were uniquely detected by xF liquid biopsy
18% were detected solely in tissue by xT
18% were detected solely in tissue by xT
ESR1 variants were more likely to be identified when analyzing cfDNA (cfDNA-only or concurrent) than tissue samples alone
Figure 2
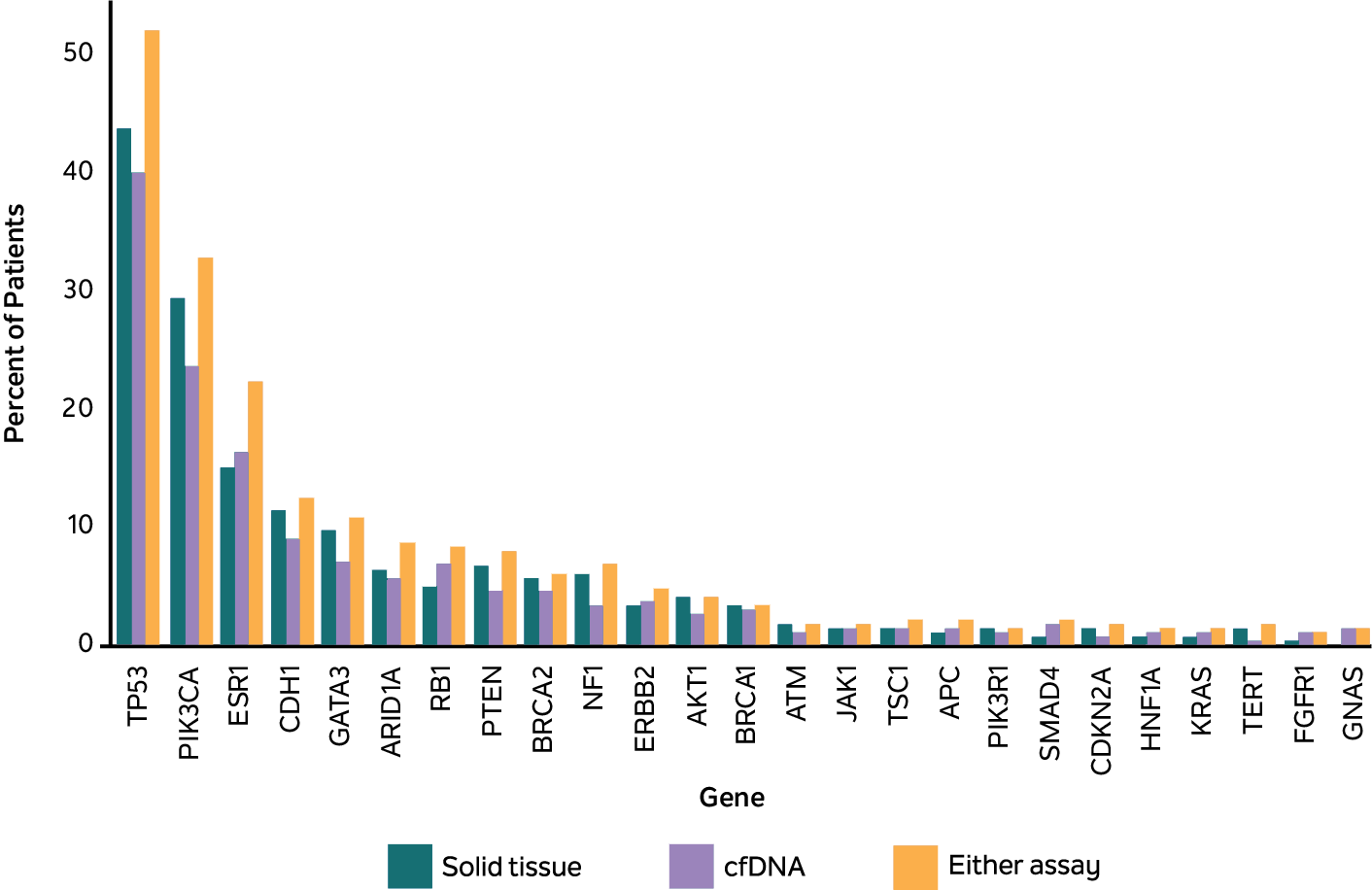
Shown are the % of MBC patients with ≥1 of the top 25 most common pathogenic variants in cfDNA (green), tissue (blue), and either test (purple), as assessed using the Tempus xF, xT, or xF/xT assay and the Tempus LENS platform.
Liu MC, et al. JCO Precis Oncol. 2022;6(1):e2100321.
READ THE MANUSCRIPT
-
07/01/2025
Transforming R&D with Generative AI and Real-World Data
Mike Yasiejko, General Manager and Executive Vice President at Tempus, recently spoke with Andrew Mazar, PhD, Chief Operating Officer at Actuate Therapeutics, about the ways Tempus’ liquid biopsy (xF+) and methylation assays have been valuable to Actuate’s work and how the company has benefitted from the partnership
Watch now -
06/12/2025
AI & ML in action: Demonstrating real-world impact in trial design & patient care
Discover how the Tempus platform leverages AI and ML to inform standard of care practices through health equity guidelines and drive insights that help refine clinical trial design. Engage with live demonstrations showcasing how our tools identify patients by modifying inclusion/exclusion criteria and leveraging patient queries. Explore how our tools integrate NCCN guidelines and empower life science teams to access current, actionable patient-journey insights. Learn how these real-world applications can drive progress in your clinical development initiatives.
Watch replay
Secure your recording now. -
06/09/2025
Bridging the translational gap: The role of organoids in oncology R&D
This white paper explores the evolving role of organoids in oncology R&D, highlighting their potential as predictive preclinical models and their ability to reduce translational risk. Download for a comprehensive overview of the scientific landscape, key adoption barriers, emerging innovations, and how pharma companies leverage organoids to accelerate precision medicine.
Read more