-
PROVIDERS
Register now
Are you getting the full picture? A webinar series on the power of comprehensive intelligent diagnostics
-
LIFE SCIENCES
REGISTER NOW
AI & ML in action: Demonstrating real-world impact in trial design & patient careThursday, June 12, 2025
11 AM PT / 1 PM CT /
2 PM ET -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
- INVESTORS
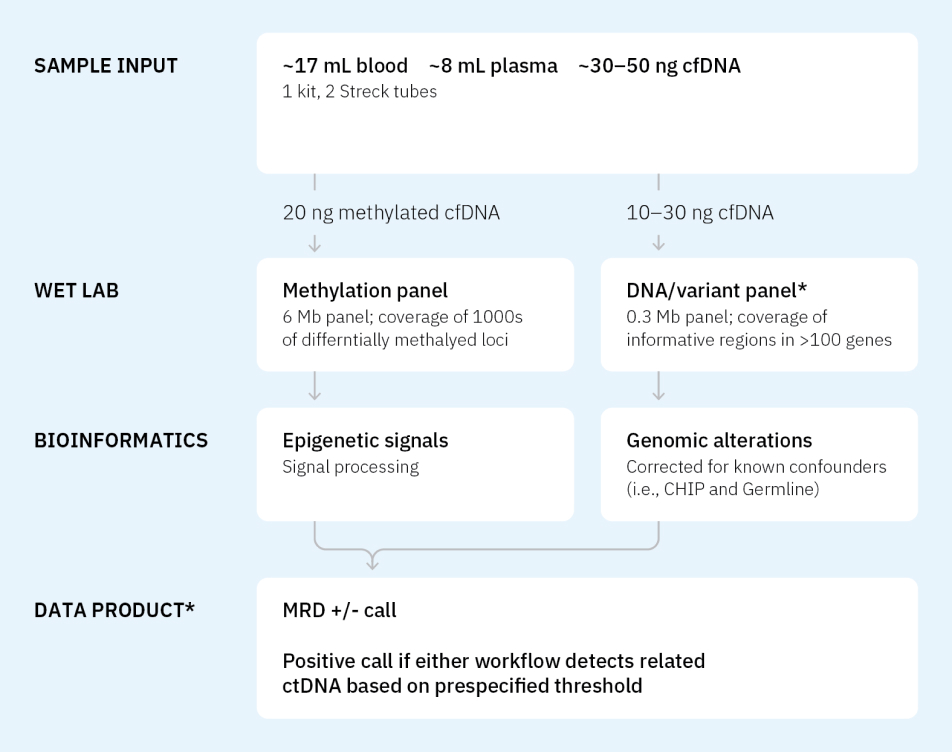
Tempus’ comprehensive MRD testing portfolio
Tempus’ comprehensive MRD testing portfolio
Tempus xM MRD
A tumor-naïve MRD assay that leverages dual methylation- and variant-based workflows to detect ctDNA and call MRD+/-.
Learn morexM (NeXT Personal®)
A tumor-informed MRD assay that utilizes whole genome sequencing to identify up to 1800 somatic variants.
Learn more
TEMPUS xM MRD
A liquid-only approach to MRD assessment
xM MRD leverages both variant and methylation workflows to call MRD+/-, bringing forward variant-level data about your patient cohort.1
Our Science
Longitudinal clinical performance of a novel tumor-naïve minimal residual disease assay in resected stage II and III colorectal cancer patients: A subset analysis from the GALAXY study in CIRCULATE-JapanxM MRD results predict disease-free survival (DFS) nearly 5 times superior to standard of care carcinoembryonic antigen (CEA) (adjusted hazard ratio 9.69 vs. 2.13).
LEARN MORE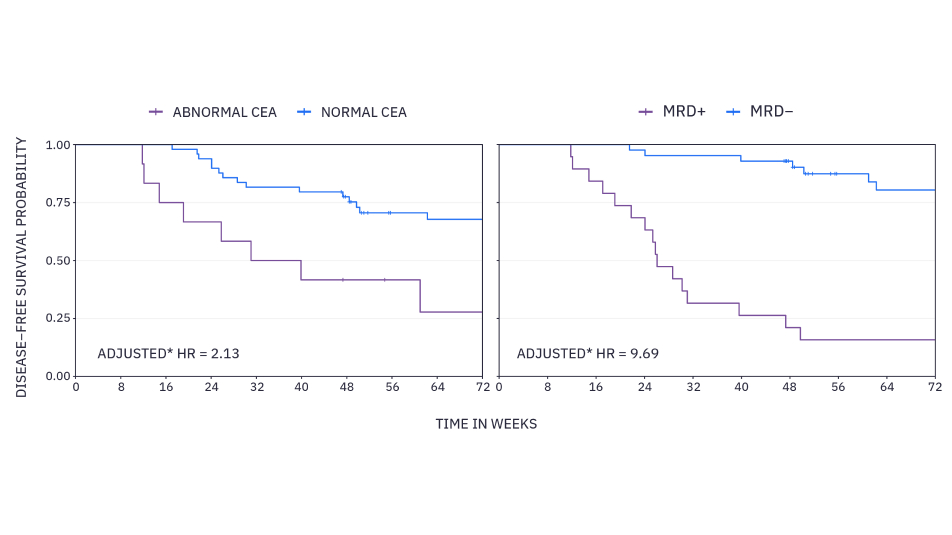
Colorectal Cancer (CRC)
Tempus xM is analytically validated2 for early stage (II and III) colorectal cancer and reports on robust variant gene data specific to this indication.2
Custom models
For indications outside of colorectal cancer, we are excited to collaborate with biopharma partners and build custom models.
IN PARTNERSHIP WITH PERSONALIS
xM (NeXT Personal®)
A tumor-informed approach to MRD assessment that delivers rapid results from one blood draw to help detect residual disease or recurrence. As an ultra sensitive, whole genome assay, xM (NeXT Personal®) enables detection of ctDNA at low levels to monitor residual disease and recurrence. Must be bundled with Tempus products and services.
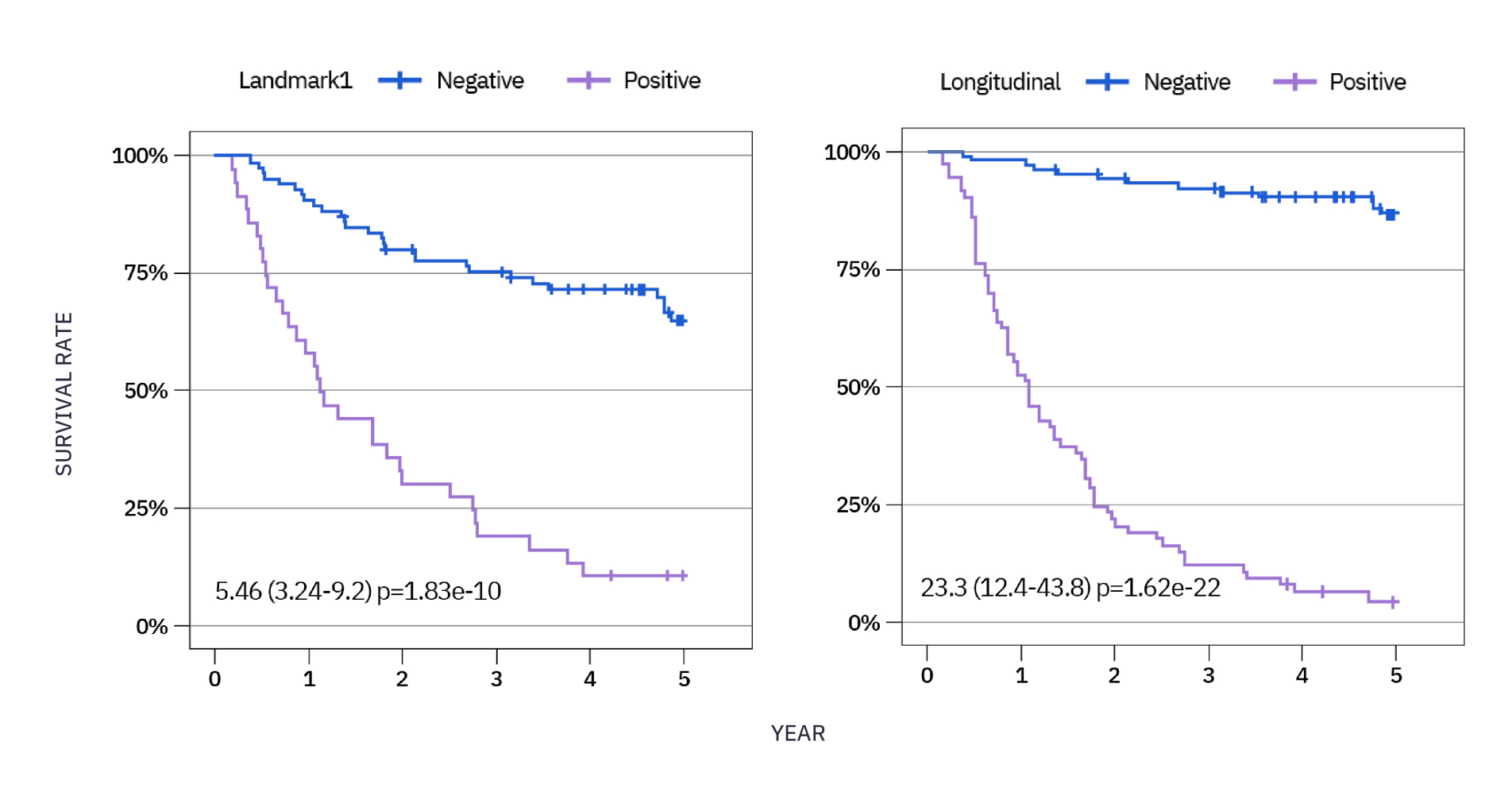
Learn more about our partnershipxM (NeXT PERSONAL® Dx) CLINICAL PERFORMANCE IN NSCLC3
As observed in a study in early stage NSCLC over a 5 year period, 85% of patients who recurred had ctDNA detected, with a median lead time of ~6 months ahead of clinical relapse.***
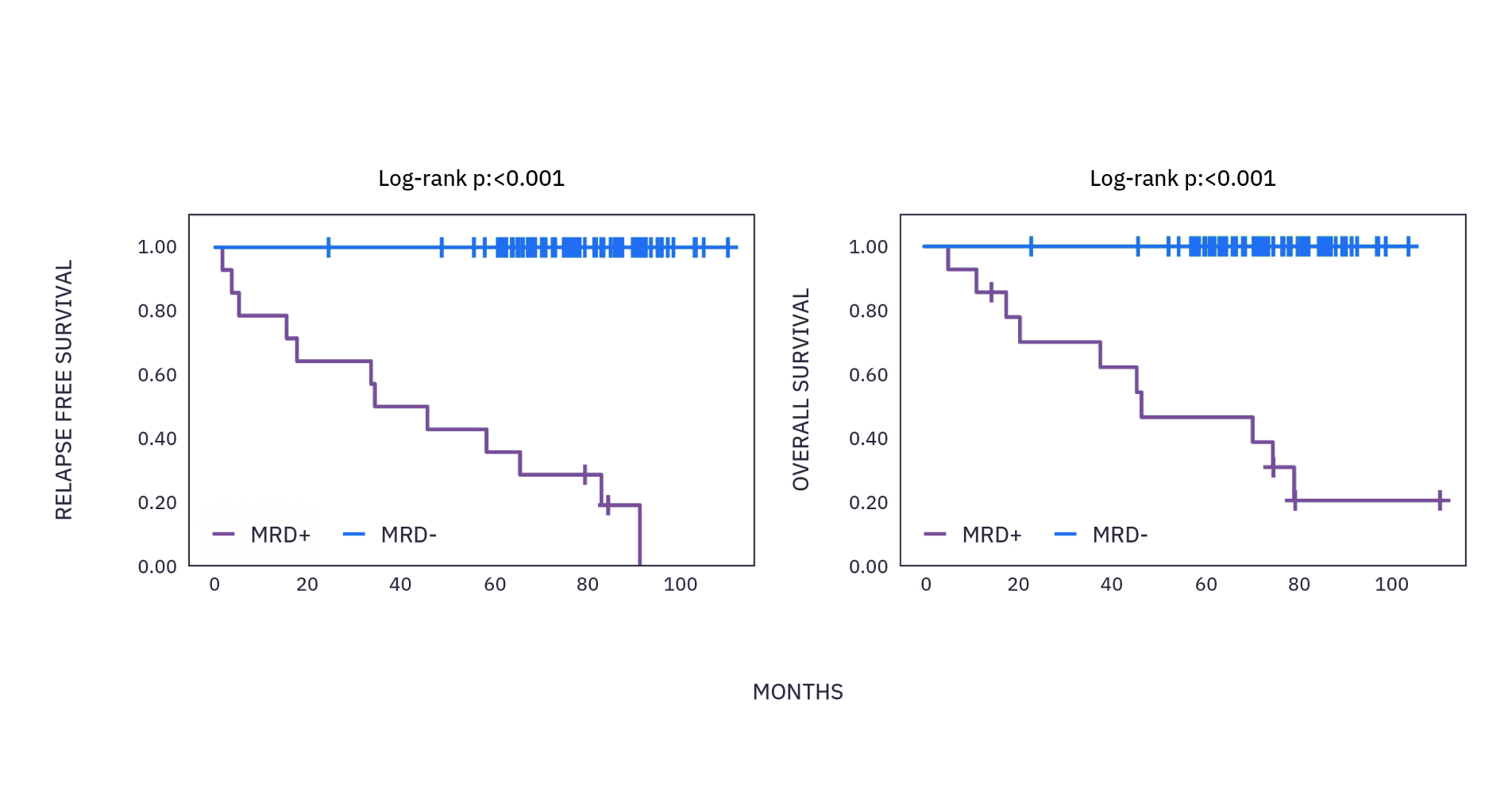
xM (NeXT PERSONAL® Dx) CLINICAL PERFORMANCE IN BREAST CANCER4
As observed in a study in early stage breast cancer over a 6 year period, >99% of patients who recurred had ctDNA detected with a median lead time of ~15 months ahead of clinical relapse.*
ctDNA Suite
Tempus offers a comprehensive portfolio for MRD and monitoring, which includes a liquid and tissue based assay to support drug development, including patient stratification for future trials, clinical trial design, and treatment response monitoring. Tempus xM Monitor (ctDNA tumor fraction calculation & monitoring) is currently available for biopharma partners for research use only.
-
TEMPUS xM MONITOR
ctDNA Tumor Fraction Calculation & Monitoring
A ctDNA assay which measures changes in ctDNA tumor fraction to provide information on early response to immunotherapy for patients with advanced cancers. Currently available for research use only.
learn MORE -
TEMPUS xF+
523 Gene Liquid Assay
A ctDNA seq panel that interrogates 523 genes focused on oncogenic and resistance mutations in cell-free DNA (cfDNA) to help clinicians identify treatment opportunities. Identifies SNV, insertions / deletions, CNVs, gene rearrangements from select genes, as well as MSI and bTMB.
learn MORE -
TEMPUS xF
105 Gene Liquid Assay
A ctDNA seq panel that detects oncogenic drivers and resistance mutations, assesses for MSI, and identifies SNVs, INDELs, and CNVs.
learn MORE -
TEMPUS xM MRD
Tumor-naïve Minimal Residual Disease Detection
An assay that detects ctDNA in patients following curative-intent treatment to identify patients at high risk of recurrence. Currently available in early stage (II and III) colorectal cancer.
learn MORE -
TEMPUS xM (NeXT PERSONAL®)
Tumor-informed Minimal Residual Disease Detection
A whole genome assay that enables detection of ctDNA at very low levels to monitor residual disease. In partnership with Personalis.
learn MORE
ctDNA Tumor Fraction Calculation & Monitoring
A ctDNA assay which measures changes in ctDNA tumor fraction to provide information on early response to immunotherapy for patients with advanced cancers. Currently available for research use only.
learn MORE523 Gene Liquid Assay
A ctDNA seq panel that interrogates 523 genes focused on oncogenic and resistance mutations in cell-free DNA (cfDNA) to help clinicians identify treatment opportunities. Identifies SNV, insertions / deletions, CNVs, gene rearrangements from select genes, as well as MSI and bTMB.
learn MORE105 Gene Liquid Assay
A ctDNA seq panel that detects oncogenic drivers and resistance mutations, assesses for MSI, and identifies SNVs, INDELs, and CNVs.
learn MORETumor-naïve Minimal Residual Disease Detection
An assay that detects ctDNA in patients following curative-intent treatment to identify patients at high risk of recurrence. Currently available in early stage (II and III) colorectal cancer.
learn MORETumor-informed Minimal Residual Disease Detection
A whole genome assay that enables detection of ctDNA at very low levels to monitor residual disease. In partnership with Personalis.
learn MORE- Dual methylation and variant workflows are utilized at the landmark timepoint (LMT) only, all subsequent timepoints utilize the methylation workflow only.
- Nakamura, Y., et al. A tumor-uninformed ctDNA assay detecting MRD in patients with resected stage II or III colorectal cancer predicts recurrence: Subset analysis from the GALAXY study in CIRCULATE-Japan. Presented at ASCO GI 2024.
- Black JRM, et al. An ultra-sensitive and specific ctDNA assay provides novel pre-operative disease stratification in early stage lung cancer. Ann Oncol. 2023;34(Suppl):S1294-S1294.
- Garcia-Murillas I, et al. Ultra-sensitive ctDNA mutation tracking to identify molecular residual disease and predict relapse in early breast cancer patients. Presented at: ASCO Annual Meeting; June 2, 2024; Chicago, IL.
* Data listed is longitudinal data. Longitudinal data encompasses landmark data and refers to a minimum of one MRD test performed post-surgery.
Trusted by hundreds of biopharma to power drug development
The information on this page is intended for life sciences companies and focuses on research and development applications.
This is AI-enabled precision medicine
This is the future of healthcare.