-
PROVIDERS
Read more
Browse our latest selection of abstracts, manuscripts, and presentations.
-
LIFE SCIENCES
LIVE WEBINAR
Optimizing research with RWD: How Lens accelerates time to insights in drug discovery.
Register now. -
PATIENTS
It's About Time
View the Tempus vision.
- RESOURCES
-
ABOUT US
View Job Postings
We’re looking for people who can change the world.
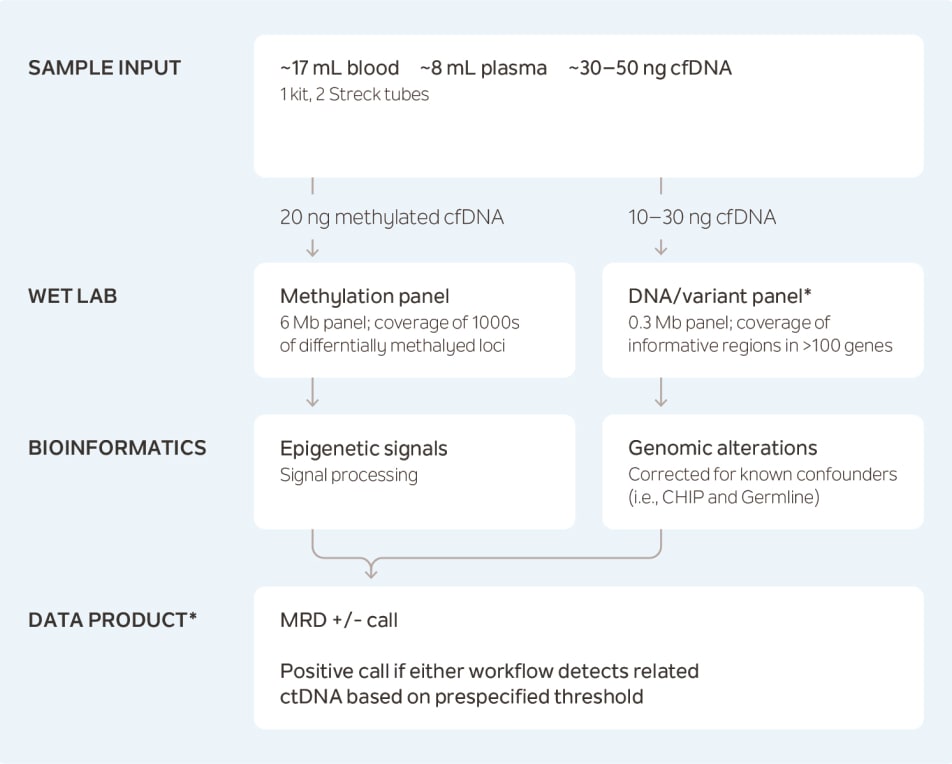
Tempus xM MRD (RUO For Biopharma)
Tumor-naïve MRD assay for ctDNA detection
xM MRD is a liquid-only approach to MRD assessment that detects trace amounts of residual ctDNA, leveraging both methylation and genomic variant MRD classifiers while applying algorithms that support filtering of artifacts, like CHIP and germline variants, to deliver a binary MRD call.
As a tumor-naïve assay with dual workflows, Tempus xM MRD offers several advantages to biopharma, including accelerated turnaround times as well as the availability of variant-level information. xM MRD is currently available for research use only (RUO) in stage II-III colorectal cancer. Tempus is actively seeking partners for indication expansion for this assay.
SOPHISTICATED MRD MODEL
Harnessing the power of a dual workflow
xM MRD leverages both variant and methylation workflows to call MRD+/-, bringing forward variant-level data about your patient cohort with every blood draw.
Faster TAT
As a tumor-naïve assay, xM MRD reduces operational research delays due to tissue procurement.
Data-informed model
The xM MRD algorithmic model was developed using Tempus’ robust multimodal database, leveraging known tumor profiles and clinical outcomes to correct for CHIP and germline confounders.
Valuable insights with every sample
xM MRD utilizes both methylation and variant workflows to call MRD+/-, helping to bring forward variant level data on cohorts with every sample.
Our Science
A tumor-uninformed ctDNA assay detecting MRD in patients with resected stage II or III colorectal cancer predicts recurrence: Subset analysis from the GALAXY study in CIRCULATE-JapanThe adjusted median disease-free survival at the landmark 1-month post-surgery time point for MRD+ patients is 39.3 weeks (9.8 months) versus >72 weeks (18 months) for xM MRD- patients, a clinically meaningful difference (Adj. HR 5.09).1
LEARN MORE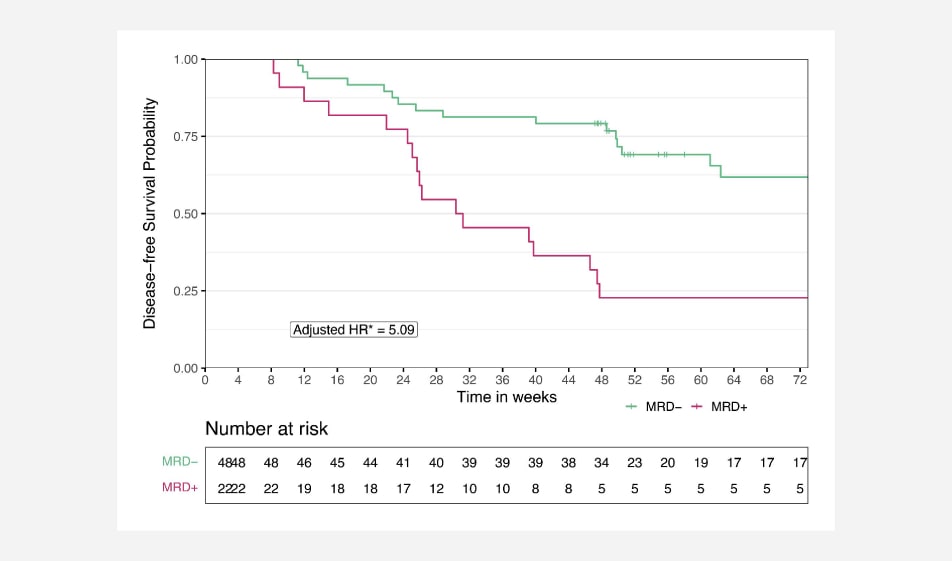
Parternship Opportunities
Strategic validation studies with flexible opportunities for additional indications
Colorectal Cancer (CRC)
Tempus xM MRD is analytically validated2 for early stage (II and III) colorectal cancer and reports on robust variant gene data specific to this indication.
Custom models
For indications outside of colorectal cancer, we are excited to collaborate with biopharma partners and build custom models.
ctDNA Suite
Tempus offers a comprehensive liquid portfolio to support drug development, including patient stratification for future trials, clinical trial design, and treatment response monitoring. Tempus xM Monitor (ctDNA tumor fraction calculation & monitoring) and Tempus xM MRD (MRD detection) are currently available for biopharma partners for research use only.
-
TEMPUS xM MRD
MRD Detection
A tumor-naïve assay that detects ctDNA in patients following curative-intent treatment to identify patients at high risk of recurrence. Currently available for research use only in colorectal cancer.
LEARN MORE -
TEMPUS xM MONITOR
ctDNA Tumor Fraction Calculation and Monitoring
A ctDNA assay which measures changes in ctDNA tumor fraction to provide information on early response to immunotherapy for patients with advanced cancers. Currently available for research use only.
LEARN MORE -
TEMPUS xF+
523 Gene Liquid Assay
A ctDNA seq panel that interrogates 523 genes focused on oncogenic and resistance mutations in cell-free DNA (cfDNA) to help clinicians identify treatment opportunities. Identifies SNV, insertions / deletions, CNVs, gene rearrangements from select genes, as well as MSI and bTMB.
LEARN MORE -
TEMPUS xF
105 Gene Liquid Assay
A ctDNA seq panel that detects oncogenic drivers and resistance mutations, assesses for MSI, and identifies SNVs, INDELs, and CNVs.
LEARN MORE
MRD Detection
A tumor-naïve assay that detects ctDNA in patients following curative-intent treatment to identify patients at high risk of recurrence. Currently available for research use only in colorectal cancer.
LEARN MOREctDNA Tumor Fraction Calculation and Monitoring
A ctDNA assay which measures changes in ctDNA tumor fraction to provide information on early response to immunotherapy for patients with advanced cancers. Currently available for research use only.
LEARN MORE523 Gene Liquid Assay
A ctDNA seq panel that interrogates 523 genes focused on oncogenic and resistance mutations in cell-free DNA (cfDNA) to help clinicians identify treatment opportunities. Identifies SNV, insertions / deletions, CNVs, gene rearrangements from select genes, as well as MSI and bTMB.
LEARN MORE105 Gene Liquid Assay
A ctDNA seq panel that detects oncogenic drivers and resistance mutations, assesses for MSI, and identifies SNVs, INDELs, and CNVs.
LEARN MORERUO Use Cases
The assay can be utilized for:
- Retrospective analysis of clinical samples from an existing or previously closed clinical study and/or
- Exploratory endpoint analysis within an existing clinical study or a clinical study still to be opened
For both of these use cases, xM MRD can help support the following research, dependent on the study design:
Read more about xM MRD RUO- Drug Development and Efficacy Monitoring
- Inform MRD Based Patient Stratification for Future Clinical Trials
- Treatment Response Assessment
- Longitudinal Disease Surveillance
- Disease Recurrence and Progression Studies
- Exploration of Therapeutic Resistance Mechanisms
- Pharmacodynamic Assessments
- Early Intervention Opportunities
- Companion Diagnostics Development
1 Nakamura, Y., et al. A tumor-uninformed ctDNA assay detecting MRD in patients with resected stage II or III colorectal cancer predicts recurrence: Subset analysis from the GALAXY study in CIRCULATE-Japan. Presented at ASCO GI 2024.
2 Statistically powered clinical validation study from GALAXY (Stage II-IV) is underway.
This is data-driven precision medicine
This is the future of healthcare.